Medicinal Chemistry ( IF 2.3 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573406416666200611103039 Mahboob Ali 1 , Momin Khan 1 , Khair Zaman 1 , Abdul Wadood 2 , Maryam Iqbal 2 , Aftab Alam 1 , Sana Shah 1 , Ashfaq Ur Rehman 3 , Muhammad Yousaf 1 , Rafaila Rafique 4 , Khalid Mohammed Khan 4
|
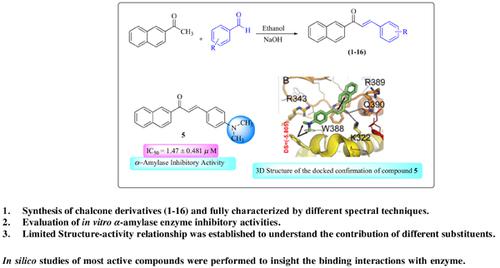
Background: The inhibition of α-amylase enzyme is one of the best therapeutic approach for the management of type II diabetes mellitus. Chalcone possesses a wide range of biological activities.
Objective: In the current study chalcone derivatives (1-16) were synthesized and evaluated their inhibitory potential against α-amylase enzyme.
Methods: For that purpose, a library of substituted (E)-1-(naphthalene-2-yl)-3-phenylprop-2-en-1-ones was synthesized by Claisen-Schmidt condensation reaction of 2-acetonaphthanone and substituted aryl benzaldehyde in the presence of base and characterized via different spectroscopic techniques such as EI-MS, HRESI-MS, 1H-, and 13C-NMR.
Results: Sixteen synthetic chalcones were evaluated for in vitro porcine pancreatic α-amylase inhibition. All the chalcones demonstrated good inhibitory activities in the range of IC50 = 1.25 ± 1.05 to 2.40 ± 0.09 μM as compared to the standard commercial drug acarbose (IC50 = 1.34 ± 0.3 μM).
Conclusion: Chalcone derivatives (1-16) were synthesized, characterized, and evaluated for their α- amylase inhibition. SAR revealed that electron donating groups in the phenyl ring have more influence on enzyme inhibition. However, to insight the participation of different substituents in the chalcones on the binding interactions with the α-amylase enzyme, in silico (computer simulation) molecular modeling analyses were carried out.
中文翻译:

查尔酮:作为有效的 α-淀粉酶抑制剂;合成、体外和计算机研究
背景:抑制α-淀粉酶是治疗II型糖尿病的最佳治疗方法之一。查尔酮具有广泛的生物活性。
目的:在目前的研究中,合成了查耳酮衍生物 (1-16) 并评估了它们对 α-淀粉酶的抑制潜力。
方法:为此,通过 2-乙酰萘酮和取代芳基的 Claisen-Schmidt 缩合反应合成了取代 (E)-1-(naphthalene-2-yl)-3-phenylprop-2-en-1-ones 库苯甲醛在碱存在下并通过不同的光谱技术表征,如 EI-MS、HRESI-MS、1 H-和13 C-NMR。
结果:评估了 16 种合成查耳酮对体外猪胰腺 α-淀粉酶的抑制作用。与标准商业药物阿卡波糖 (IC 50 = 1.34 ± 0.3 μM)相比,所有查耳酮在 IC 50 = 1.25 ± 1.05 至 2.40 ± 0.09 μM范围内均表现出良好的抑制活性。
结论: 查尔酮衍生物 (1-16) 已合成、表征并评估其 α-淀粉酶抑制。SAR显示苯环中的给电子基团对酶抑制作用更大。然而,为了深入了解查耳酮中不同取代基对与 α-淀粉酶的结合相互作用的参与,进行了计算机模拟(计算机模拟)分子建模分析。


























 京公网安备 11010802027423号
京公网安备 11010802027423号