Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.jechem.2021.08.069 Shiqing Hu 1, 2 , Huan Li 1, 2 , Xue Dong 1 , Zhongwei Cao 1 , Bingjie Pang 1 , Liming Zhang 1, 2 , Wenguang Yu 3 , Jianping Xiao 1, 2 , Xuefeng Zhu 1, 2 , Weishen Yang 1, 2
|
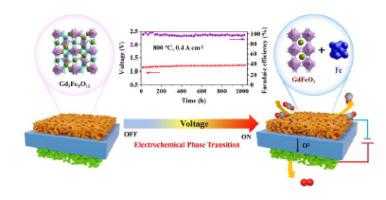
CO2 electroreduction reaction (CO2RR), combined with solid oxide electrolysis cells (SOECs), is a feasible technology for the storage of renewable electric energy, while its development is limited by the catalytic activity and stability on cathodes. Here, a novel garnet oxide (Gd3Fe5O12) cathode is designed, where the garnet oxide is converted to perovskite oxide and iron via in situ electrochemical phase transition during CO2 electroreduction, resulting in high activity with Faradaic efficiency close to 100% and great stability over 1000 h galvanostatic test. A variety of experimental characterizations and density functional theory calculations indicate that in situ exsolved Fe clusters can effectively enhance the adsorption energies of intermediates and lowering the CO2 dissociation barriers. Microkinetic modelling confirms that CO2RR goes through a dissociative adsorption mechanism and the electronic transfer for CO2 dissociation is the rate-determining step.
中文翻译:

Gd3Fe5O12:通过原位电化学相变合理设计CO2电还原阴极
CO 2电还原反应(CO 2 RR)结合固体氧化物电解槽(SOECs)是一种可行的可再生电能存储技术,但其发展受到阴极催化活性和稳定性的限制。在这里,设计了一种新型石榴石氧化物 (Gd 3 Fe 5 O 12 ) 阴极,其中石榴石氧化物通过CO 2期间的原位电化学相变转化为钙钛矿氧化物和铁电还原,导致法拉第效率接近 100% 的高活性和超过 1000 小时恒电流测试的稳定性。各种实验表征和密度泛函理论计算表明,原位溶出的Fe团簇可以有效提高中间体的吸附能并降低CO 2解离势垒。微动力学模型证实 CO 2 RR 经历解离吸附机制,CO 2解离的电子转移是速率决定步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号