Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.jechem.2021.08.068 Xiaotong Jin 1 , Xialiang Li 1 , Haitao Lei 1 , Kai Guo 1 , Bin Lv 1 , Hongbo Guo 1 , Dandan Chen 1 , Wei Zhang 1 , Rui Cao 1
|
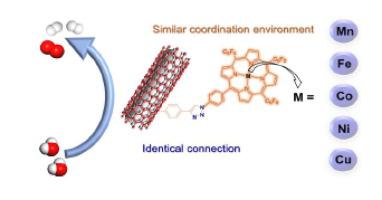
Developing cheap and efficient electrocatalysts for water splitting is required for energy conversion techniques. Many first-row transition metal complexes have been shown to be active for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Metal ions play crucial roles in these catalytic processes, but the activity dependence on the nature of metal ions has been rarely studied due to the difficulty to compare metal complexes with different coordination environments. We herein reported the synthesis of a series of metal complexes of azido-substituted porphyrin (1), in which metal ions have very similar coordination environments. By grafting 1-M (M = Mn, Fe, Co, Ni, and Cu) onto alkyne-functionalized carbon nanotubes (CNTs) through the same covalent connection, the resulted hybrids 1-M@CNT were all active and robust for both electrocatalytic HER and OER in alkaline aqueous solutions. Among these hybrids, 1-Fe@CNT displayed the highest electrocatalytic activity for HER, while 1-Co@CNT was the most active one for OER. Moreover, a two-electrode water electrolysis cell assembled with 1-Fe@CNT as the cathode and 1-Co@CNT as the anode required smaller applied bias potential by 210 mV to get 10 mA/cm2 current density as compared to that assembled with Pt/C and Ir/C with the same amount of metal loading. This work is significant to correlate HER and OER activity with the nature of first-row transition metal ions and to highlight promising potential applications of molecular electrocatalysis in water splitting.
中文翻译:

比较具有相似配位环境的第一排过渡金属配合物的电催化析氢和析氧活性
能量转换技术需要开发廉价且高效的水分解电催化剂。许多第一排过渡金属配合物已被证明对析氢反应 (HER) 和析氧反应 (OER) 具有活性。金属离子在这些催化过程中起着至关重要的作用,但由于难以比较具有不同配位环境的金属配合物,因此很少研究活性对金属离子性质的依赖性。我们在此报道了一系列叠氮基取代卟啉金属配合物的合成 (1),其中金属离子具有非常相似的配位环境。通过相同的共价连接将 1-M(M = Mn、Fe、Co、Ni 和 Cu)接枝到炔烃官能化的碳纳米管 (CNT) 上,所得杂化物 1-M@CNT 对于碱性水溶液中的电催化 HER 和 OER 均具有活性和稳健性。在这些杂化物中,1-Fe@CNT 对 HER 的电催化活性最高,而 1-Co@CNT 对 OER 的电催化活性最高。此外,以 1-Fe@CNT 为阴极和 1-Co@CNT 为阳极的双电极水电解槽需要较小的外加偏压 210 mV 才能获得 10 mA/cm2与具有相同金属负载量的 Pt/C 和 Ir/C 组装的电流密度相比。这项工作对于将 HER 和 OER 活性与第一排过渡金属离子的性质相关联,并突出分子电催化在水分解中的潜在应用具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号