Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.jechem.2021.08.070 Samuel Bertolini 1 , Timo Jacob 1, 2, 3
|
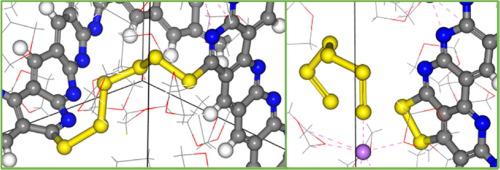
Lithium-sulfur battery (LSB) represents an important candidate to be used in energy storage applications, due to its high specific capacities. Sulfurized-polyacrylonitrile (SPAN) is a candidate as a host material in LSB to replace graphite, due to its ability to chemisorb polysulfides (PSs). The sulfur chains attached to the polymer can reversibly form Li2S, and SPAN indicates to have a good cyclability and better performance than graphite, thus, SPAN acts partially as an active and also as a host material. In this study, we investigated the capacity of the solvent or the SPAN to lose a hydrogen atom from the backbone, to predict possible anodic reactions between solvent and host material. The simulation suggests that the photophilic salts may preferentially react with the solvent, and possibly building a cathode electrolyte interphase (CEI). We observed that an undercoordinated carbon (Cuc) can be thermodynamically created, due to lithiation. The Cuc can react with the solvent on the polymer backbone through different mechanisms, however, the simulations indicated that the reaction should be affected by the interaction between the solvent and Cuc, according to SPAN’s configuration. Moreover, Cuc reacts with long sulfur chains attached to SPAN, capturing sulfur and forming a C-S bond. A sulfur chain from one SPAN can connect to another polymer backbone, however, this process is affected by lithiation and vice-versa. Therefore, this work also investigates the formation of interconnected SPAN structures and the multiple Cuc effects.
中文翻译:

锂硫电池中的硫化聚丙烯腈:低配位碳与聚合物结构之间的相互作用
由于其高比容量,锂硫电池 (LSB) 是用于储能应用的重要候选者。硫化聚丙烯腈 (SPAN) 是 LSB 中替代石墨的主体材料的候选材料,因为它具有化学吸附多硫化物 (PS) 的能力。连接在聚合物上的硫链可以可逆地形成 Li 2S 和 SPAN 表明具有良好的循环性和比石墨更好的性能,因此,SPAN 部分充当活性材料,也充当主体材料。在这项研究中,我们研究了溶剂或 SPAN 从主链上失去一个氢原子的能力,以预测溶剂和主体材料之间可能发生的阳极反应。模拟表明,亲光盐可能优先与溶剂反应,并可能构建阴极电解质界面 (CEI)。我们观察到,由于锂化,可以通过热力学产生未配位的碳 (C uc )。的C UC可以通过不同的机制与聚合物骨架上的溶剂反应,然而,根据 SPAN 的配置,模拟表明反应应该受到溶剂和 C uc之间相互作用的影响。此外,C uc与连接到 SPAN 的长硫链反应,捕获硫并形成 CS 键。来自一个 SPAN 的硫链可以连接到另一个聚合物主链,但是,这个过程会受到锂化的影响,反之亦然。因此,这项工作还研究了互连 SPAN 结构的形成和多重 C uc效应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号