Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.colsurfa.2021.127526 Zuoli Li 1 , Anirban Chakraborty 1 , Jessica Fuentes 2 , Edgar Zamora 2 , Flavio Vázquez 2 , Zhenghe Xu 1 , Qingxia Liu 1 , César Flores 2 , William C. McCaffrey 1
|
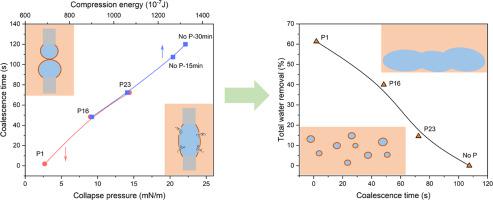
Stabilization mechanisms of water-in-oil emulsions have been well studied. The process of dehydration with demulsifiers in these previous works have focused primarily on macro scale bottle tests or on microscale investigations of asphaltene film. A connection between the two scales, however, is necessary to better understand the mechanisms of dehydration using demulsifiers. In our present work, results from bottle tests of undiluted crude oil were compared with the coalescence process of water droplets in solvent diluted crude oil and with visualization experiments of the interfacial film rigidity through observing the crumpling of water droplets in diluted crude oil. To gain further insight into the molecular interactions between the native surface active materials and three different polymeric demulsifiers at the solvent-water interface, Langmuir interfacial isotherms were measured using diluted crude oil-water systems with and without the addition of demulsifiers. The highest water removal percentage was obtained though the addition of a random biopolymer demulsifier, butyl acrylate/2-(dimethylamino) ethyl acrylate (P1), that also rendered the shortest coalescence time of the three polymers tested, indicating the importance of coalescence behavior in dehydration. In addition, the smallest crumpling ratio which corresponds to lowest rigidity was detected for the P1 treated interface. For the first time, we observed that the diluted crude oil-water interfacial film collapsed easily upon compression with the administration of the very efficient demulsifier P1. It was found that the collapse pressure can be used as a measure of the rigidity of very soft interfacial films that may not be measurable directly by compression energy. Based on the close relation between the dehydration process and the interaction between demulsifiers and natural active surfactants, it can be deduced that, efficient demulsifiers essentially disrupt the continuity of the asphaltenic interfacial films and reduce the strength of anchoring of these natural surfactants at the interface, allowing for the collapse of segments and resulting in efficient dehydration.
中文翻译:

原油脱水油水界面破乳剂原油相互作用研究
油包水乳液的稳定机制已经得到很好的研究。在这些以前的工作中,使用破乳剂脱水的过程主要集中在宏观瓶试验或沥青质膜的微观研究上。然而,两个尺度之间的联系对于更好地理解使用破乳剂的脱水机制是必要的。在我们目前的工作中,未稀释原油的瓶装试验结果与溶剂稀释原油中水滴的聚结过程进行了比较,并通过观察稀释原油中水滴的皱缩与界面膜刚度的可视化实验进行了比较。为了进一步了解天然表面活性材料与溶剂-水界面处三种不同聚合物破乳剂之间的分子相互作用,Langmuir 界面等温线是使用稀释的原油-水系统在添加和不添加破乳剂的情况下测量的。通过添加无规生物聚合物破乳剂丙烯酸丁酯/丙烯酸 2-(二甲氨基)乙酯 (P1) 获得了最高的除水百分比,这也使测试的三种聚合物的聚结时间最短,表明聚结行为在脱水。此外,对于 P1 处理的界面,检测到对应于最低刚度的最小起皱率。我们第一次观察到稀释的原油-水界面膜在使用非常有效的破乳剂 P1 后在压缩时很容易坍塌。发现塌陷压力可用作非常软的界面膜的刚度的量度,其可能无法通过压缩能直接测量。根据脱水过程与破乳剂与天然活性表面活性剂相互作用的密切关系,可以推断,高效破乳剂本质上破坏了沥青质界面膜的连续性,降低了这些天然表面活性剂在界面处的锚固强度,允许片段坍塌并导致有效脱水。


























 京公网安备 11010802027423号
京公网安备 11010802027423号