当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Enzyme Cascade Catalyzed Asymmetrization of Primary Alcohols: Synthesis of Enantiocomplementary Chiral β-Nitroalcohols
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-08 , DOI: 10.1002/adsc.202100803 Ayon Chatterjee 1 , D.H. Sreenivasa Rao 1 , Santosh Padhi 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-08 , DOI: 10.1002/adsc.202100803 Ayon Chatterjee 1 , D.H. Sreenivasa Rao 1 , Santosh Padhi 2
Affiliation
|
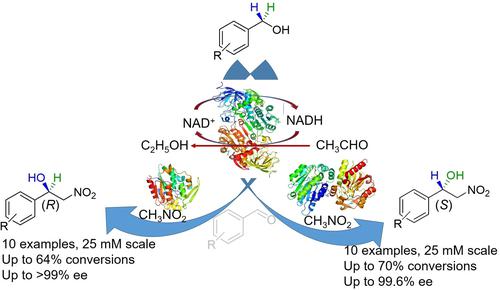
Biocatalytic asymmetrization of inexpensive and stable primary alcohols to prepare enantioenriched β-nitroalcohols is an important development in green chemistry for the production of chiral pharmaceutical intermediates. Herein, we report a one-pot, two-step cascade reaction sequence in which first benzylic alcohols were oxidized to produce corresponding benzaldehydes using horse liver alcohol dehydrogenase (HLADH). The in situ generated aldehydes were then reacted in a biphasic medium with nitromethane by Arabidopsis thaliana hydroxynitrile lyase (AtHNL) or Baliospermum montanum HNL (BmHNL) catalyzed Henry reaction to produce stereoselective β-nitroalcohols with (R) or (S) configuration, respectively. Using HLADH-AtHNL, (R)-β-nitroalcohols were obtained in up to 64% conversion, and HLADH-BmHNL, (S)-β-nitroalcohols in up to 70% conversion, while in both cases excellent stereoselectivity (up to >99% ee) was achieved. The concept was proven by functionalization of sp3 C−H bond of ten simple achiral benzylic alcohols to enantiocomplementary chiral β-nitroalcohols.
中文翻译:

一锅酶级联催化伯醇不对称化:对映互补手性 β-硝基醇的合成
廉价且稳定的伯醇的生物催化不对称化制备对映体富集的 β-硝基醇是生产手性药物中间体的绿色化学的重要进展。在此,我们报告了一个一锅两步级联反应序列,其中使用马肝醇脱氢酶 (HLADH) 氧化第一个苯甲醇以产生相应的苯甲醛。然后原位生成的醛在双相培养基中与硝基甲烷通过拟南芥羟基腈裂解酶 ( At HNL) 或Baliospermum montanum HNL ( Bm HNL) 催化亨利反应生成立体选择性 β-硝基醇与 ( R ) 或 ( S) 配置。使用 HLADH- At HNL,( R )-β-硝基醇的转化率高达 64%,而 HLADH- Bm HNL,( S )-β-硝基醇的转化率高达 70%,同时在这两种情况下都具有出色的立体选择性(高达达到 >99% ee )。通过将十种简单的非手性苯甲醇的sp 3 CH 键官能化为对映互补手性 β-硝基醇,证明了这一概念。
更新日期:2021-09-08
中文翻译:

一锅酶级联催化伯醇不对称化:对映互补手性 β-硝基醇的合成
廉价且稳定的伯醇的生物催化不对称化制备对映体富集的 β-硝基醇是生产手性药物中间体的绿色化学的重要进展。在此,我们报告了一个一锅两步级联反应序列,其中使用马肝醇脱氢酶 (HLADH) 氧化第一个苯甲醇以产生相应的苯甲醛。然后原位生成的醛在双相培养基中与硝基甲烷通过拟南芥羟基腈裂解酶 ( At HNL) 或Baliospermum montanum HNL ( Bm HNL) 催化亨利反应生成立体选择性 β-硝基醇与 ( R ) 或 ( S) 配置。使用 HLADH- At HNL,( R )-β-硝基醇的转化率高达 64%,而 HLADH- Bm HNL,( S )-β-硝基醇的转化率高达 70%,同时在这两种情况下都具有出色的立体选择性(高达达到 >99% ee )。通过将十种简单的非手性苯甲醇的sp 3 CH 键官能化为对映互补手性 β-硝基醇,证明了这一概念。


























 京公网安备 11010802027423号
京公网安备 11010802027423号