Applied Geochemistry ( IF 3.4 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.apgeochem.2021.105091 Jiaxi Zhang 1, 2 , Xu Ma 3 , Shaofeng Wang 1 , Mario A. Gomez 4 , Shuhua Yao 4 , Yongfeng Jia 1
|
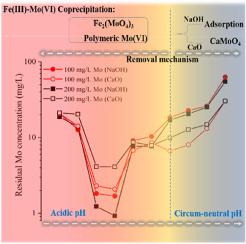
Coprecipitation of molybdate (Mo(VI)) with ferric iron (Fe(III)) is usually employed for the treatment of Mo-bearing acid mine drainage (AMD) and mineral-processing effluents. However, the speciation of Mo(VI) in the Fe(III)–Mo(VI) coprecipitates and the roles of process parameters in Mo(VI) removal remain unclear. In this work, the effects of pH, neutralization reagents (CaO vs NaOH) and co-ions (Zn2+, Cu2+ and Ni2+) on the removal and speciation of Mo(VI) were investigated. It was found that Mo(VI) removal was significantly enhanced at circum-neutral pH by using CaO as base instead of NaOH or in the presence of Ni2+ ions. X-ray diffraction, Fourier transform infrared, Raman and linear combination fitting of Mo K-edge X-ray absorption near edge structure (XANES) spectra results indicated that amorphous ferric molybdate was the major Mo(VI) phase in the coprecipitates formed at acidic pH regardless of the base used. While at circum-neutral pH, Mo(VI) mainly existed as surface adsorbed form on ferrihydrite in NaOH neutralized coprecipitates, and as calcium molybdate in CaO neutralized coprecipitates. The pre-edge features of Mo K-edge and L3-edge XANES features indicated the formation of polymeric molybdate in the acidic coprecipitates. This work may have implications to Mo(VI) immobilization via coprecipitation with Fe(III) and the fate of Mo(VI) in tailings.
中文翻译:

pH、中和剂和共离子对 Fe(III)-Mo(VI) 共沉淀过程中 Mo(VI) 去除和形态形成的影响
钼酸盐 (Mo(VI)) 与三价铁 (Fe(III)) 的共沉淀通常用于处理含钼的酸性矿山排水 (AMD) 和矿物加工废水。然而,Fe(III)-Mo(VI) 共沉淀物中 Mo(VI) 的形态以及工艺参数在 Mo(VI) 去除中的作用仍不清楚。在这项工作中,研究了 pH、中和剂(CaO 与 NaOH)和共离子(Zn 2+、Cu 2+和 Ni 2+)对 Mo(VI) 去除和形态形成的影响。结果表明,在中性 pH 值下,使用 CaO 代替 NaOH 或在 Ni 2+存在下可显着提高 Mo(VI) 去除率离子。X 射线衍射、傅里叶变换红外、拉曼和 Mo K 边 X 射线吸收近边结构 (XANES) 光谱结果的线性组合拟合表明无定形钼酸铁是酸性条件下形成的共沉淀物中的主要 Mo(VI) 相。 pH 值与使用的碱无关。而在环中性 pH 值下,Mo(VI) 主要以 NaOH 中和的共沉淀物中的水铁矿表面吸附形式存在,并在 CaO 中和的共沉淀物中以钼酸钙的形式存在。Mo K 边缘和 L 3边缘 XANES 特征的前边缘特征表明在酸性共沉淀物中形成聚合钼酸盐。这项工作可能对 Mo(VI) 通过与 Fe(III) 的共沉淀和尾矿中 Mo(VI) 的归宿产生影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号