当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polycation-Mediated Transfection: Mechanisms of Internalization and Intracellular Trafficking
Biomacromolecules ( IF 6.2 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.biomac.1c00697 Bryn D Monnery 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.biomac.1c00697 Bryn D Monnery 1
Affiliation
|
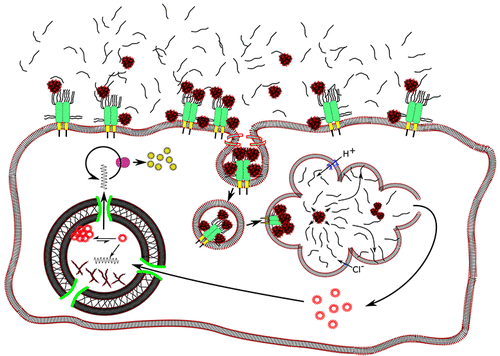
Polyplex-mediated gene transfection is now in its’ fourth decade of serious research, but the promise of polyplex-mediated gene therapy has yet to fully materialize. Only approximately one in a million applied plasmids actually expresses. A large part of this is due to an incomplete understanding of the mechanism of polyplex transfection. There is an assumption that internalization must follow a canonical mechanism of receptor mediated endocytosis. Herein, we present arguments that untargeted (and most targeted) polyplexes do not utilize these routes. By incorporating knowledge of syndecan-polyplex interactions, we can show that syndecans are the “target” for polyplexes. Further, it is known that free polycations (which disrupt cell-membranes by acid-catalyzed hydrolysis of phospholipid esters) are necessary for (untargeted) endocytosis. This can be incorporated into the model to produce a novel mechanism of endocytosis, which fits the observed phenomenology. After membrane translocation, polyplex containing vesicles reach the endosome after diffusing through the actin mesh below the cell membrane. From there, they are acidified and trafficked toward the lysosome. Some polyplexes are capable of escaping the endosome and unpacking, while others are not. Herein, it is argued that for some polycations, as acidification proceeds the polyplexes excluding free polycations, which disrupt the endosomal membrane by acid-catalyzed hydrolysis, allowing the polyplex to escape. The polyplex’s internal charge ratio is now insufficient for stability and it releases plasmids which diffuse to the nucleus. A small proportion of these plasmids diffuse through the nuclear pore complex (NPC), with aggregation being the major cause of loss. Those plasmids that have diffused through the NPC will also aggregate, and this appears to be the reason such a small proportion of nuclear plasmids express mRNA. Thus, the structural features which promote unpacking in the endosome and allow for endosomal escape can be determined, and better polycations can be designed.
中文翻译:

聚阳离子介导的转染:内化和细胞内运输的机制
Polyplex 介导的基因转染现在已进入其认真研究的第四个十年,但 Polyplex 介导的基因治疗的前景尚未完全实现。只有大约百万分之一的应用质粒实际表达。这在很大程度上是由于对复合物转染机制的不完全了解。有一种假设,即内化必须遵循受体介导的内吞作用的典型机制。在此,我们提出非靶向(和大多数靶向)复合体不利用这些途径的论点。通过结合 Syndecan-Polyplex 相互作用的知识,我们可以证明 Syndecan 是 Polyplex 的“目标”。此外,已知游离聚阳离子(通过磷脂酯的酸催化水解破坏细胞膜)对于(非靶向)内吞作用是必需的。这可以结合到模型中以产生一种新的内吞作用机制,它符合观察到的现象学。膜易位后,含有囊泡的复合物通过细胞膜下方的肌动蛋白网扩散后到达内体。从那里,它们被酸化并被运送到溶酶体。一些复合物能够逃离内体并解包,而另一些则不能。在本文中,有人认为,对于某些聚阳离子,随着酸化的进行,除游离聚阳离子之外的复合物会通过酸催化水解破坏内体膜,从而使复合物逃逸。复合物的内部电荷比现在不足以稳定,它会释放扩散到细胞核的质粒。这些质粒的一小部分通过核孔复合物(NPC)扩散,聚集是损失的主要原因。那些通过 NPC 扩散的质粒也会聚集,这似乎是如此少比例的核质粒表达 mRNA 的原因。因此,可以确定促进内体解包并允许内体逃逸的结构特征,并且可以设计更好的聚阳离子。
更新日期:2021-10-12
中文翻译:

聚阳离子介导的转染:内化和细胞内运输的机制
Polyplex 介导的基因转染现在已进入其认真研究的第四个十年,但 Polyplex 介导的基因治疗的前景尚未完全实现。只有大约百万分之一的应用质粒实际表达。这在很大程度上是由于对复合物转染机制的不完全了解。有一种假设,即内化必须遵循受体介导的内吞作用的典型机制。在此,我们提出非靶向(和大多数靶向)复合体不利用这些途径的论点。通过结合 Syndecan-Polyplex 相互作用的知识,我们可以证明 Syndecan 是 Polyplex 的“目标”。此外,已知游离聚阳离子(通过磷脂酯的酸催化水解破坏细胞膜)对于(非靶向)内吞作用是必需的。这可以结合到模型中以产生一种新的内吞作用机制,它符合观察到的现象学。膜易位后,含有囊泡的复合物通过细胞膜下方的肌动蛋白网扩散后到达内体。从那里,它们被酸化并被运送到溶酶体。一些复合物能够逃离内体并解包,而另一些则不能。在本文中,有人认为,对于某些聚阳离子,随着酸化的进行,除游离聚阳离子之外的复合物会通过酸催化水解破坏内体膜,从而使复合物逃逸。复合物的内部电荷比现在不足以稳定,它会释放扩散到细胞核的质粒。这些质粒的一小部分通过核孔复合物(NPC)扩散,聚集是损失的主要原因。那些通过 NPC 扩散的质粒也会聚集,这似乎是如此少比例的核质粒表达 mRNA 的原因。因此,可以确定促进内体解包并允许内体逃逸的结构特征,并且可以设计更好的聚阳离子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号