当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Significant Loop Motions in the SsoPTP Protein Tyrosine Phosphatase Allow for Dual General Acid Functionality
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.biochem.1c00365 Justin Pinkston 1 , Jihye Jo 2 , Keith J Olsen 1 , Drake Comer 1 , Charsti A Glaittli 1 , J Patrick Loria 2, 3 , Sean J Johnson 1 , Alvan C Hengge 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.biochem.1c00365 Justin Pinkston 1 , Jihye Jo 2 , Keith J Olsen 1 , Drake Comer 1 , Charsti A Glaittli 1 , J Patrick Loria 2, 3 , Sean J Johnson 1 , Alvan C Hengge 1
Affiliation
|
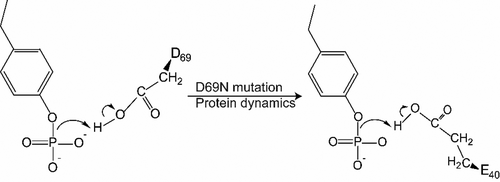
Conformational dynamics are important factors in the function of enzymes, including protein tyrosine phosphatases (PTPs). Crystal structures of PTPs first revealed the motion of a protein loop bearing a conserved catalytic aspartic acid, and subsequent nuclear magnetic resonance and computational analyses have shown the presence of motions, involved in catalysis and allostery, within and beyond the active site. The tyrosine phosphatase from the thermophilic and acidophilic Sulfolobus solfataricus (SsoPTP) displays motions of its acid loop together with dynamics of its phosphoryl-binding P-loop and the Q-loop, the first instance of such motions in a PTP. All three loops share the same exchange rate, implying their motions are coupled. Further evidence of conformational flexibility comes from mutagenesis, kinetics, and isotope effect data showing that E40 can function as an alternate general acid to protonate the leaving group when the conserved acid, D69, is mutated to asparagine. SsoPTP is not the first PTP to exhibit an alternate general acid (after VHZ and TkPTP), but E40 does not correspond to the sequence or structural location of the alternate general acids in those precedents. A high-resolution X-ray structure with the transition state analogue vanadate clarifies the role of the active site arginine R102, which varied in structures of substrates bound to a catalytically inactive mutant. The coordinated motions of all three functional loops in SsoPTP, together with the function of an alternate general acid, suggest that catalytically competent conformations are present in solution that have not yet been observed in crystal structures.
中文翻译:

SsoPTP 蛋白酪氨酸磷酸酶中的显着环运动允许双通用酸功能
构象动力学是酶功能的重要因素,包括蛋白质酪氨酸磷酸酶 (PTP)。PTP 的晶体结构首先揭示了带有保守催化天冬氨酸的蛋白质环的运动,随后的核磁共振和计算分析表明,在活性位点内外存在涉及催化和变构的运动。来自嗜热和嗜酸硫叶菌的酪氨酸磷酸酶(SsoPTP) 显示其酸环的运动及其磷酸结合 P 环和 Q 环的动力学,这是 PTP 中此类运动的第一个实例。所有三个循环共享相同的汇率,这意味着它们的运动是耦合的。构象灵活性的进一步证据来自诱变、动力学和同位素效应数据,这些数据表明,当保守酸 D69 突变为天冬酰胺时,E40 可以作为替代通用酸使离去基团质子化。SsoPTP 不是第一个展示替代通用酸的 PTP(在 VHZ 和 TkPTP 之后),但 E40 不对应于这些先例中替代通用酸的序列或结构位置。具有过渡态类似物钒酸盐的高分辨率 X 射线结构阐明了活性位点精氨酸 R102 的作用,其与催化失活的突变体结合的底物结构不同。SsoPTP 中所有三个功能环的协调运动,以及替代通用酸的功能,表明溶液中存在催化能力的构象,但在晶体结构中尚未观察到。
更新日期:2021-09-28
中文翻译:

SsoPTP 蛋白酪氨酸磷酸酶中的显着环运动允许双通用酸功能
构象动力学是酶功能的重要因素,包括蛋白质酪氨酸磷酸酶 (PTP)。PTP 的晶体结构首先揭示了带有保守催化天冬氨酸的蛋白质环的运动,随后的核磁共振和计算分析表明,在活性位点内外存在涉及催化和变构的运动。来自嗜热和嗜酸硫叶菌的酪氨酸磷酸酶(SsoPTP) 显示其酸环的运动及其磷酸结合 P 环和 Q 环的动力学,这是 PTP 中此类运动的第一个实例。所有三个循环共享相同的汇率,这意味着它们的运动是耦合的。构象灵活性的进一步证据来自诱变、动力学和同位素效应数据,这些数据表明,当保守酸 D69 突变为天冬酰胺时,E40 可以作为替代通用酸使离去基团质子化。SsoPTP 不是第一个展示替代通用酸的 PTP(在 VHZ 和 TkPTP 之后),但 E40 不对应于这些先例中替代通用酸的序列或结构位置。具有过渡态类似物钒酸盐的高分辨率 X 射线结构阐明了活性位点精氨酸 R102 的作用,其与催化失活的突变体结合的底物结构不同。SsoPTP 中所有三个功能环的协调运动,以及替代通用酸的功能,表明溶液中存在催化能力的构象,但在晶体结构中尚未观察到。



























 京公网安备 11010802027423号
京公网安备 11010802027423号