Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.jhazmat.2021.127169 Yan Wang 1 , Lilin Xiong 2 , Xiaoquan Huang 3 , Ying Ma 3 , Lingyue Zou 3 , Ying Liang 3 , Wenjing Xie 3 , Yongya Wu 3 , Xiaoru Chang 3 , Zhihui Wang 3 , Meng Tang 3
|
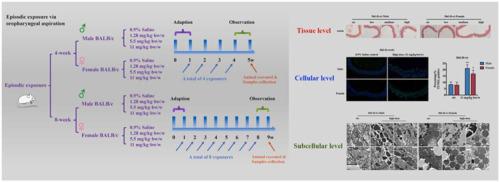
Airborne particulate matter (PM) has been linked to cardiovascular diseases, but the underlying mechanisms remain unclear, especially at realistic exposure levels. In this study, both male and female BALB/c mice were employed to assess vascular homeostasis following a standard urban particulate matter, PM SRM1648a, via oropharyngeal aspiration at three environmentally relevant concentrations. The tested indicators included histopathological observation and lipid deposition, as well as redox biology and inflammatory responses. Furthermore, endothelial monolayer, vascular cell apoptosis and subcellular function were assessed to decipher whether episodic PM SRM1648a exposure leads to vascular damage after multiple periods of treatment, including subacute (4 weeks) and subchronic (8 weeks) durations. As a result, PM aspiration caused thickening of airways, leukocytes infiltration and adhesion to alveoli, with the spot of particles engulfed by pulmonary macrophages. Meanwhile, it induced local and systemic oxidative stress and inflammation, but limited pathological changes were captured throughout aortic tissues after either subacute or subchronic treatment. Furthermore, even in the absence of aortic impairment, vascular cell equilibrium has been disturbed by the characteristics of endothelial monolayer disintegration and cell apoptosis. Mechanistically, PM SRM1648a activated molecular markers of ER stress (BIP) and mitochondrial dynamics (DRP1) at both transcriptional and translational levels, which were strongly correlated to ox-inflammation and could serve as early checkpoints of hazardous events. In summary, our data basically indicate that episodic exposure of BALB/c mice to PM SRM1648a exerts limited effects on vascular histopathological alterations, but induces vascular cell apoptosis and subcellular dysfunction, to which local and systemic redox biology and inflammation are probably correlated.
中文翻译:

通过环境相关浓度的多终点评估,间歇性暴露于空气中的颗粒物会导致 BALB/c 小鼠的亚细胞功能障碍和主动脉细胞损伤
空气中的颗粒物 (PM) 与心血管疾病有关,但其潜在机制仍不清楚,尤其是在实际暴露水平下。在这项研究中,雄性和雌性 BALB/c 小鼠都被用来评估标准城市颗粒物 PM SRM1648a 后的血管稳态,通过三个环境相关浓度的口咽抽吸。测试指标包括组织病理学观察和脂质沉积,以及氧化还原生物学和炎症反应。此外,评估内皮单层、血管细胞凋亡和亚细胞功能,以破译在多个治疗期(包括亚急性(4 周)和亚慢性(8 周)持续时间)后,偶发性 PM SRM1648a 暴露是否会导致血管损伤。因此,PM 吸入导致气道增厚、白细胞浸润和粘附于肺泡,颗粒点被肺巨噬细胞吞噬。同时,它诱导局部和全身氧化应激和炎症,但在亚急性或亚慢性治疗后,整个主动脉组织的病理变化有限。此外,即使在没有主动脉损伤的情况下,血管细胞平衡也受到内皮单层崩解和细胞凋亡的特征的干扰。从机制上讲,PM SRM1648a 在转录和翻译水平上激活了 ER 应激 (BIP) 和线粒体动力学 (DRP1) 的分子标记,这与氧化炎症密切相关,可以作为危险事件的早期检查点。总之,我们的数据基本上表明,BALB/c 小鼠间歇性暴露于 PM SRM1648a 对血管组织病理学改变的影响有限,但会诱导血管细胞凋亡和亚细胞功能障碍,这可能与局部和全身氧化还原生物学和炎症相关。内皮单层崩解和细胞凋亡的特点扰乱了血管细胞平衡。从机制上讲,PM SRM1648a 在转录和翻译水平上激活了 ER 应激 (BIP) 和线粒体动力学 (DRP1) 的分子标记,这与氧化炎症密切相关,可以作为危险事件的早期检查点。总之,我们的数据基本上表明,BALB/c 小鼠间歇性暴露于 PM SRM1648a 对血管组织病理学改变的影响有限,但会诱导血管细胞凋亡和亚细胞功能障碍,这可能与局部和全身氧化还原生物学和炎症相关。内皮单层崩解和细胞凋亡的特点扰乱了血管细胞平衡。从机制上讲,PM SRM1648a 在转录和翻译水平上激活了 ER 应激 (BIP) 和线粒体动力学 (DRP1) 的分子标记,这与氧化炎症密切相关,可以作为危险事件的早期检查点。总之,我们的数据基本上表明,BALB/c 小鼠间歇性暴露于 PM SRM1648a 对血管组织病理学改变的影响有限,但会诱导血管细胞凋亡和亚细胞功能障碍,这可能与局部和全身氧化还原生物学和炎症相关。PM SRM1648a 在转录和翻译水平上激活了 ER 应激 (BIP) 和线粒体动力学 (DRP1) 的分子标记,这与氧化炎症密切相关,可以作为危险事件的早期检查点。总之,我们的数据基本上表明,BALB/c 小鼠间歇性暴露于 PM SRM1648a 对血管组织病理学改变的影响有限,但会诱导血管细胞凋亡和亚细胞功能障碍,这可能与局部和全身氧化还原生物学和炎症相关。PM SRM1648a 在转录和翻译水平上激活了 ER 应激 (BIP) 和线粒体动力学 (DRP1) 的分子标记,这与氧化炎症密切相关,可以作为危险事件的早期检查点。总之,我们的数据基本上表明,BALB/c 小鼠间歇性暴露于 PM SRM1648a 对血管组织病理学改变的影响有限,但会诱导血管细胞凋亡和亚细胞功能障碍,这可能与局部和全身氧化还原生物学和炎症相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号