当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of bicyclic vinyl triazenes by Ficini-type reactions
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01546j Carl Thomas Bormann 1 , Farzaneh Fadaei-Tirani 1 , Rosario Scopelliti 1 , Kay Severin 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01546j Carl Thomas Bormann 1 , Farzaneh Fadaei-Tirani 1 , Rosario Scopelliti 1 , Kay Severin 1
Affiliation
|
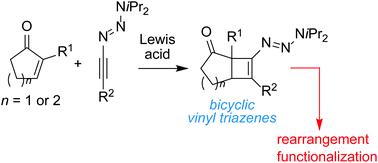
Cyclic olefins with triazene functions can display interesting reactivity, but synthetic access to these compounds is limited thus far. Herein, we describe the synthesis of cyclobutenyl triazenes fused to cyclopentanone or cyclohexanone rings. The bicyclic compounds are obtained by Lewis acid-catalyzed [2 + 2] cycloaddition reactions of 1-alkynyl triazenes and enones. In the presence of Me2AlCl, bicyclic [4.2.0] triazenes rearrange into [3.2.1] ring systems. The triazene function in the latter can be used for further functionalizations. Notably, we show that vinyl triazenes can serve as substrates for Pd-catalyzed cross-coupling reactions with arylboronic acids.
中文翻译:

Ficini型反应合成双环乙烯基三氮烯
具有三氮烯官能团的环状烯烃可以表现出有趣的反应性,但到目前为止,合成这些化合物的途径有限。在此,我们描述了与环戊酮或环己酮环稠合的环丁烯基三氮烯的合成。双环化合物是通过路易斯酸催化的 1-炔基三氮烯和烯酮的 [2 + 2] 环加成反应获得的。在 Me 2 AlCl 的存在下,双环 [4.2.0] 三氮烯重排成 [3.2.1] 环系统。后者中的三氮烯官能团可用于进一步的官能化。值得注意的是,我们表明乙烯基三氮烯可以作为 Pd 催化的与芳基硼酸的交叉偶联反应的底物。
更新日期:2021-09-09
中文翻译:

Ficini型反应合成双环乙烯基三氮烯
具有三氮烯官能团的环状烯烃可以表现出有趣的反应性,但到目前为止,合成这些化合物的途径有限。在此,我们描述了与环戊酮或环己酮环稠合的环丁烯基三氮烯的合成。双环化合物是通过路易斯酸催化的 1-炔基三氮烯和烯酮的 [2 + 2] 环加成反应获得的。在 Me 2 AlCl 的存在下,双环 [4.2.0] 三氮烯重排成 [3.2.1] 环系统。后者中的三氮烯官能团可用于进一步的官能化。值得注意的是,我们表明乙烯基三氮烯可以作为 Pd 催化的与芳基硼酸的交叉偶联反应的底物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号