Journal of Structural Biology ( IF 3 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.jsb.2021.107796 Sebastian Fuchs 1 , Alexey G Kikhney 2 , Robin Schubert 3 , Charlotte Kaiser 4 , Eva Liebau 4 , Dmitri I Svergun 2 , Christian Betzel 1 , Markus Perbandt 1
|
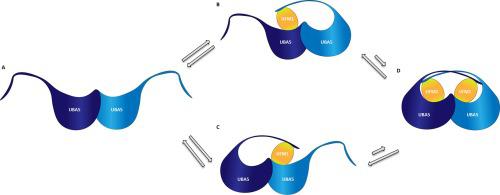
Ubiquitin fold modifier 1 (UFM1) is an ubiquitin-like protein (Ubl) involved especially in endoplasmic stress response. Activation occurs via a three-step mechanism like other Ubls. Data obtained reveal that UFM1 regulates the oligomeric state of ubiquitin activating enzyme 5 (UBA5) to initiate the activation step. Mixtures of homodimers and heterotrimers are observed in solution at the equilibrium state, demonstrating that the UBA5-UFM1 complex undergoes several concentration dependent oligomeric translational states to form a final functional complex. The oligomerization state of unbound UBA5 is also concentration dependent and shifts from the monomeric to the dimeric state. Data describing different oligomeric states are complemented with binding studies that reveal a negative cooperativity for the complex formation and thereby provide more detailed insights into the complex formation mechanism.
中文翻译:

UBA5-UFM1复合物形成的结构和动力学显示了对UBA5激活机制的新见解
泛素折叠修饰剂 1 (UFM1) 是一种泛素样蛋白 (Ubl),尤其参与内质应激反应。激活通过与其他 Ubl 一样的三步机制发生。获得的数据表明,UFM1 调节泛素激活酶 5 (UBA5) 的寡聚状态以启动激活步骤。在平衡状态的溶液中观察到同源二聚体和异源三聚体的混合物,表明 UBA5-UFM1 复合物经历了几种浓度依赖性低聚翻译状态以形成最终的功能复合物。未结合的 UBA5 的低聚状态也依赖于浓度,并从单体状态转变为二聚状态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号