当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NaFSI and NaTFSI Solutions in Ether Solvents from Monoglyme to Poly(ethylene oxide)—A Molecular Dynamics Study
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.jpcb.1c05793 Piotr Wróbel 1 , Piotr Kubisiak 1 , Andrzej Eilmes 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.jpcb.1c05793 Piotr Wróbel 1 , Piotr Kubisiak 1 , Andrzej Eilmes 1
Affiliation
|
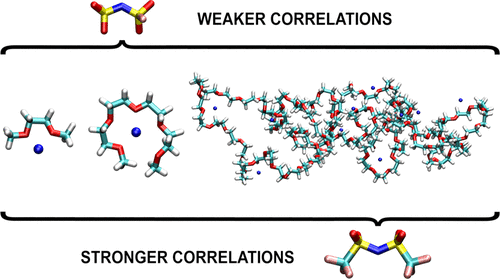
Classical molecular dynamics simulations have been performed for a series of electrolytes based on sodium bis(fluorosulfonyl)imide or sodium bis(trifluoromethylsulfonyl)imide salts and monoglyme, tetraglyme, and poly(ethylene oxide) as solvents. Structural properties have been assessed through the analysis of coordination numbers and binding patterns. Residence times for Na–O interactions have been used to investigate the stability of solvation shells. Diffusion coefficients of ions and electrical conductivity of the electrolytes have been estimated from molecular dynamics trajectories. Contributions to the total conductivity have been analyzed in order to investigate the role of ion–ion correlations. It has been found that the anion–cation interactions are more probable in the systems with NaTFSI salts. Accordingly, the degree of correlations between ion motions is larger in NaTFSI-based electrolytes.
中文翻译:

从单甘醇二甲醚到聚环氧乙烷的醚溶剂中的 NaFSI 和 NaTFSI 溶液——分子动力学研究
已经对基于双(氟磺酰基)亚胺钠或双(三氟甲基磺酰基)亚胺钠盐以及单甘醇二甲醚、四甘醇二甲醚和聚(环氧乙烷)作为溶剂的一系列电解质进行了经典分子动力学模拟。结构特性已通过对配位数和结合模式的分析进行评估。Na-O 相互作用的停留时间已被用于研究溶剂化壳的稳定性。离子的扩散系数和电解质的电导率已从分子动力学轨迹估计。为了研究离子-离子相关性的作用,已经分析了对总电导率的贡献。已经发现阴离子-阳离子相互作用在具有 NaTFSI 盐的系统中更有可能发生。因此,
更新日期:2021-09-16
中文翻译:

从单甘醇二甲醚到聚环氧乙烷的醚溶剂中的 NaFSI 和 NaTFSI 溶液——分子动力学研究
已经对基于双(氟磺酰基)亚胺钠或双(三氟甲基磺酰基)亚胺钠盐以及单甘醇二甲醚、四甘醇二甲醚和聚(环氧乙烷)作为溶剂的一系列电解质进行了经典分子动力学模拟。结构特性已通过对配位数和结合模式的分析进行评估。Na-O 相互作用的停留时间已被用于研究溶剂化壳的稳定性。离子的扩散系数和电解质的电导率已从分子动力学轨迹估计。为了研究离子-离子相关性的作用,已经分析了对总电导率的贡献。已经发现阴离子-阳离子相互作用在具有 NaTFSI 盐的系统中更有可能发生。因此,


























 京公网安备 11010802027423号
京公网安备 11010802027423号