当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Cations on the Oxidation and Atmospheric Corrosion of Iron Interfaces to Minerals
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpca.1c06451 Chathura de Alwis 1 , Mikhail Trought 1 , Julia Lundeen 2 , Kathryn A Perrine 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpca.1c06451 Chathura de Alwis 1 , Mikhail Trought 1 , Julia Lundeen 2 , Kathryn A Perrine 1
Affiliation
|
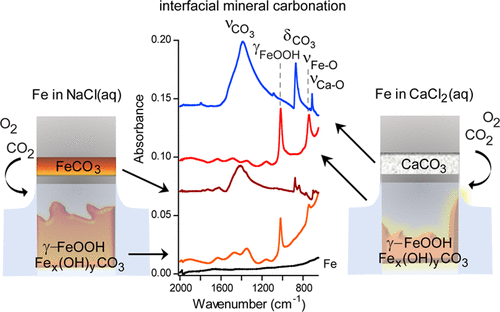
Surface corrosion involves a series of redox reactions that are catalyzed by the presence of ions. On infrastructure surfaces and in complex and natural environments, iron surfaces readily undergo redox reactions, impacting chemical processes. In this study, the effect of how cations influence the formation of the mineral scale on iron surfaces and its connection to surface corrosion was investigated in CaCl2(aq) and NaCl(aq) electrolytes. Polarized modulated-infrared reflection absorption spectroscopy (PM-IRRAS) measurements were used to measure the oxidation and formation of carbonates at the air/electrolyte/iron interface, which confirmed that the iron surface oxidized faster in CaCl2(aq) than in NaCl(aq). PM-IRRAS, attenuated total reflectance–Fourier transformed infrared spectroscopy, and X-ray photoelectron spectroscopy show that after the adsorption of atmospheric O2 and CO2, calcium carbonate (CaCO3) in the form of calcite and aragonite was produced on iron in the presence of CaCl2(aq), whereas siderite (FeCO3) was produced on the surface of iron in the presence of NaCl(aq). However, in either solution without gradual O2 and CO2 exposure, a heterogeneous mixture of lepidocrocite (γ-FeOOH) and an iron hydroxy carbonate (Fex(OH)yCO3) was grown on the iron surface. In situ liquid AFM was used to measure the surface roughness in CaCl2(aq) and NaCl(aq), as an estimation of the corrosion rate. In CaCl2(aq), Fe was found to corrode faster than Fe in NaCl(aq) due to more ions at equimolar concentrations. Surface physical changes, as measured by ex situ AFM, confirmed the presence of a heterogeneous mixture of γ-FeOOH and an Fex(OH)yCO3 in the submerged region. This indicates that the cation does not affect the type of mineral grown on the Fe surface in the region completely submerged in the electrolyte. These results suggest that the cations play a unique role in the initial stages of corrosion at the interface region, influencing the uptake of atmospheric CO2 and mineral nucleation. The knowledge gained from these interfacial reactions are important for understanding the connection between surface corrosion, mineral grown, and CO2 capture for sequestration.
中文翻译:

阳离子对铁矿物界面氧化和大气腐蚀的影响
表面腐蚀涉及一系列由离子存在催化的氧化还原反应。在基础设施表面以及复杂的自然环境中,铁表面很容易发生氧化还原反应,从而影响化学过程。在这项研究中,在 CaCl 2 (aq) 和 NaCl(aq) 电解质中研究了阳离子如何影响铁表面矿物垢形成的影响及其与表面腐蚀的联系。偏振调制红外反射吸收光谱 (PM-IRRAS) 测量用于测量空气/电解质/铁界面处碳酸盐的氧化和形成,这证实铁表面在 CaCl 2 中氧化得更快(aq) 比在 NaCl(aq) 中。PM-IRRAS、衰减全反射-傅里叶变换红外光谱和X射线光电子能谱表明,大气中的O 2和CO 2吸附后,在铁上生成方解石和文石形式的碳酸钙(CaCO 3)。 CaCl 2 (aq)的存在,而菱铁矿 (FeCO 3 ) 在 NaCl(aq) 的存在下在铁表面产生。然而,在没有逐渐接触O 2和 CO 2 的任一溶液中,纤铁矿 (γ-FeOOH) 和羟基碳酸铁 (Fe x (OH) y CO 3) 生长在铁表面。原位液体 AFM 用于测量 CaCl 2 (aq) 和 NaCl(aq) 中的表面粗糙度,作为腐蚀速率的估计。在 CaCl 2 (aq) 中,由于等摩尔浓度的离子更多,因此发现 Fe 比 NaCl(aq) 中的 Fe 腐蚀得更快。通过非原位AFM测量的表面物理变化证实存在 γ-FeOOH 和 Fe x (OH) y CO 3的异质混合物在淹没区。这表明在完全浸没在电解质中的区域中,阳离子不会影响在 Fe 表面上生长的矿物类型。这些结果表明,阳离子在界面区域腐蚀的初始阶段发挥着独特的作用,影响大气 CO 2的吸收和矿物成核。从这些界面反应中获得的知识对于理解表面腐蚀、矿物生长和用于封存的CO 2捕获之间的联系非常重要。
更新日期:2021-09-16
中文翻译:

阳离子对铁矿物界面氧化和大气腐蚀的影响
表面腐蚀涉及一系列由离子存在催化的氧化还原反应。在基础设施表面以及复杂的自然环境中,铁表面很容易发生氧化还原反应,从而影响化学过程。在这项研究中,在 CaCl 2 (aq) 和 NaCl(aq) 电解质中研究了阳离子如何影响铁表面矿物垢形成的影响及其与表面腐蚀的联系。偏振调制红外反射吸收光谱 (PM-IRRAS) 测量用于测量空气/电解质/铁界面处碳酸盐的氧化和形成,这证实铁表面在 CaCl 2 中氧化得更快(aq) 比在 NaCl(aq) 中。PM-IRRAS、衰减全反射-傅里叶变换红外光谱和X射线光电子能谱表明,大气中的O 2和CO 2吸附后,在铁上生成方解石和文石形式的碳酸钙(CaCO 3)。 CaCl 2 (aq)的存在,而菱铁矿 (FeCO 3 ) 在 NaCl(aq) 的存在下在铁表面产生。然而,在没有逐渐接触O 2和 CO 2 的任一溶液中,纤铁矿 (γ-FeOOH) 和羟基碳酸铁 (Fe x (OH) y CO 3) 生长在铁表面。原位液体 AFM 用于测量 CaCl 2 (aq) 和 NaCl(aq) 中的表面粗糙度,作为腐蚀速率的估计。在 CaCl 2 (aq) 中,由于等摩尔浓度的离子更多,因此发现 Fe 比 NaCl(aq) 中的 Fe 腐蚀得更快。通过非原位AFM测量的表面物理变化证实存在 γ-FeOOH 和 Fe x (OH) y CO 3的异质混合物在淹没区。这表明在完全浸没在电解质中的区域中,阳离子不会影响在 Fe 表面上生长的矿物类型。这些结果表明,阳离子在界面区域腐蚀的初始阶段发挥着独特的作用,影响大气 CO 2的吸收和矿物成核。从这些界面反应中获得的知识对于理解表面腐蚀、矿物生长和用于封存的CO 2捕获之间的联系非常重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号