Journal of Power Sources ( IF 9.2 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.jpowsour.2021.230388 Teklay Mezgebe Hagos , Hailemariam Kassa Bezabh , Haylay Ghidey Redda , Endalkachew Asefa Moges , Wei-Hsiang Huang , Chen-Jui Huang , Wei-Nien Su , Hongjie Dai , Bing Joe Hwang
|
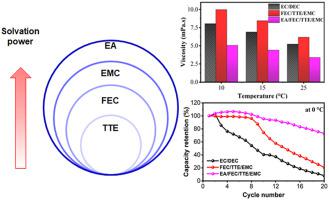
An anode-free lithium metal battery (AFLMB) configuration can be used to develop electrolytes for wide-temperature applications. The charge/discharge performance of an electrolyte consisting of lithium hexafluorophosphate (LiPF6) in a mixture of fluoroethylene carbonate (FEC), 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE), and ethyl methyl carbonate (EMC) has been is reported as an electrolyte for lithium metal batteries. It has a good passivating capability and wide electrochemical windows relative to the commercial electrolyte. Conversely, its lower ionic conductivity and high viscosity impede practical application. Hence, an electrolyte of 1 M LiPF6 in EA/FEC/TTE/EMC (2:1:5:2 by vol.) is developed by adding a quaternary solvent of ethyl acetate (EA). The electrolyte exhibits a lower viscosity and higher ionic conductivity than 1 M LiPF6 in FEC/TTE/EMC (3:5:2 by vol.). At 0 °C, 1 M LiPF6 in EA/FEC/TTE/EMC (2:1:5:2 by vol.) provides capacity retention of 30 % and the average Coulombic efficiency (av. CE) of 95 % using the Cu||NMC111 after 40 cycles at a current density of 0.2 mA/cm2. The synergy of higher ionic conductivity and formation of LiF layer in the developed electrolyte extends the service-temperature range of AFLMB. This study opens an avenue in developing low-temperature electrolytes using an AFLMB.
中文翻译:

探索碳酸盐和醚基电解质在各种条件下运行的无负极锂金属电池的性能
无阳极锂金属电池 (AFLMB) 配置可用于开发适用于宽温应用的电解质。由六氟磷酸锂 (LiPF 6 ) 在氟代碳酸亚乙酯 (FEC)、1,1,2,2-四氟乙基-2,2,3,3-四氟丙基醚 (TTE) 混合物中组成的电解液的充放电性能,据报道,碳酸甲乙酯 (EMC) 可用作锂金属电池的电解质。相对于商业电解质,它具有良好的钝化能力和较宽的电化学窗口。相反,其较低的离子电导率和高粘度阻碍了实际应用。因此,1 M LiPF 6的电解质在 EA/FEC/TTE/EMC(2:1:5:2,体积比)中,通过添加乙酸乙酯 (EA) 的季铵溶剂进行显影。该电解质在 FEC/TTE/EMC(体积比为 3:5:2)中表现出比 1 M LiPF 6更低的粘度和更高的离子电导率。在 0 °C 下,EA/FEC/TTE/EMC(体积比为 2:1:5:2)中的1 M LiPF 6提供 30% 的容量保持率和 95% 的平均库仑效率(平均 CE),使用Cu||NMC111 在 0.2 mA/cm 2的电流密度下循环 40 次。更高的离子电导率和在开发的电解质中形成 LiF 层的协同作用扩展了 AFLMB 的使用温度范围。这项研究为使用 AFLMB 开发低温电解质开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号