Redox Biology ( IF 11.4 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.redox.2021.102125 Jonathan V Dietz 1 , Mathilda M Willoughby 2 , Robert B Piel 3 , Teresa A Ross 3 , Iryna Bohovych 1 , Hannah G Addis 4 , Jennifer L Fox 4 , William N Lanzilotta 3 , Harry A Dailey 5 , James A Wohlschlegel 6 , Amit R Reddi 2 , Amy E Medlock 7 , Oleh Khalimonchuk 8
|
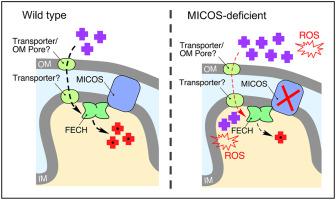
Heme is an essential cofactor required for a plethora of cellular processes in eukaryotes. In metazoans the heme biosynthetic pathway is typically partitioned between the cytosol and mitochondria, with the first and final steps taking place in the mitochondrion. The pathway has been extensively studied and its biosynthetic enzymes structurally characterized to varying extents. Nevertheless, understanding of the regulation of heme synthesis and factors that influence this process in metazoans remains incomplete. Therefore, we investigated the molecular organization as well as the physical and genetic interactions of the terminal pathway enzyme, ferrochelatase (Hem15), in the yeast Saccharomyces cerevisiae. Biochemical and genetic analyses revealed dynamic association of Hem15 with Mic60, a core component of the mitochondrial contact site and cristae organizing system (MICOS). Loss of MICOS negatively impacts Hem15 activity, affects the size of the Hem15 high-mass complex, and results in accumulation of reactive and potentially toxic tetrapyrrole precursors that may cause oxidative damage. Restoring intermembrane connectivity in MICOS-deficient cells mitigates these cytotoxic effects. These data provide new insights into how heme biosynthetic machinery is organized and regulated, linking mitochondrial architecture-organizing factors to heme homeostasis.
中文翻译:

线粒体接触位点和嵴组织系统 (MICOS) 机器通过实现铁螯合酶的最佳性能来支持血红素生物合成
血红素是真核生物中大量细胞过程所需的重要辅助因子。在后生动物中,血红素生物合成途径通常在细胞质和线粒体之间进行划分,第一步和最后一步发生在线粒体中。该途径已被广泛研究,其生物合成酶在结构上具有不同程度的特征。然而,对血红素合成的调节和影响后生动物这一过程的因素的理解仍然不完整。因此,我们研究了酵母Saccharomyces cerevisiae中末端途径酶 ferrochelatase (Hem15) 的分子组织以及物理和遗传相互作用. 生化和遗传分析揭示了 Hem15 与 Mic60 的动态关联,Mic60 是线粒体接触位点和嵴组织系统 (MICOS) 的核心组成部分。MICOS 的损失会对 Hem15 活性产生负面影响,影响 Hem15 高质量复合物的大小,并导致可能导致氧化损伤的反应性和潜在毒性四吡咯前体的积累。恢复 MICOS 缺陷细胞的膜间连接可减轻这些细胞毒性作用。这些数据提供了关于血红素生物合成机制如何组织和调节的新见解,将线粒体结构组织因子与血红素稳态联系起来。


























 京公网安备 11010802027423号
京公网安备 11010802027423号