Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2021-09-07 , DOI: 10.1016/j.bmcl.2021.128354 Tao Xiao 1 , Luxin Sun 1 , Min Zhang 1 , Zilu Li 2 , Eric B Haura 3 , Ernst Schonbrunn 1 , Haitao Ji 2
|
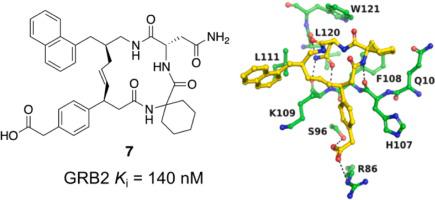
A monocarboxylic inhibitor was designed and synthesized to disrupt the protein–protein interaction (PPI) between GRB2 and phosphotyrosine-containing proteins. Biochemical characterizations show compound 7 binds with the Src homology 2 (SH2) domain of GRB2 and is more potent than EGFR1068 phosphopeptide 14-mer. X-ray crystallographic studies demonstrate compound 7 occupies the GRB2 binding site for phosphotyrosine-containing sequences and reveal key structural features for GRB2–inhibitor binding. This compound with a –1 formal charge offers a new direction for structural optimization to generate cell-permeable inhibitors for this key protein target of the aberrant Ras-MAPK signaling cascade.
中文翻译:

GRB2 SH2结构域单羧酸抑制剂的合成和结构表征
设计并合成了一种单羧酸抑制剂来破坏 GRB2 和含磷酸酪氨酸的蛋白质之间的蛋白质相互作用 (PPI)。生化表征显示化合物7与 GRB2 的 Src 同源 2 (SH2) 结构域结合,并且比 EGFR 1068磷酸肽 14-mer 更有效。X 射线晶体学研究表明,化合物7占据了含有磷酸酪氨酸序列的 GRB2 结合位点,并揭示了 GRB2 抑制剂结合的关键结构特征。这种具有 –1 正式电荷的化合物为结构优化提供了新的方向,从而为异常 Ras-MAPK 信号级联的这一关键蛋白靶点生成细胞渗透性抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号