当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanochemical N-Chlorination Reaction of Hydantoin: In Situ Real-Time Kinetic Study by Powder X-ray Diffraction and Raman Spectroscopy
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssuschemeng.1c03812 Inês C. B. Martins 1 , Maria Carta 2 , Sebastian Haferkamp 1 , Torvid Feiler 1, 3 , Francesco Delogu 2, 4, 5 , Evelina Colacino 6 , Franziska Emmerling 1, 3
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssuschemeng.1c03812 Inês C. B. Martins 1 , Maria Carta 2 , Sebastian Haferkamp 1 , Torvid Feiler 1, 3 , Francesco Delogu 2, 4, 5 , Evelina Colacino 6 , Franziska Emmerling 1, 3
Affiliation
|
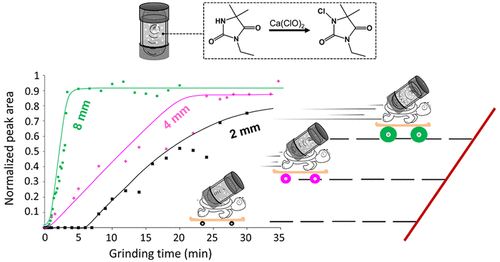
Mechanochemistry has become a valuable tool for the synthesis of new molecules, especially in the field of organic chemistry. In the present work, we investigate the kinetic profile of the chlorination reaction of N-3-ethyl-5,5-dimethylhydantoin (EDMH) activated and driven by ball milling. The reaction has been carried out using 2 mm, 4 mm, 5 mm, 6 mm, and 8 mm ball sizes in a new small custom-made Perspex milling jar. The crystal structure of the starting material EDMH and the 1-chloro-3-ethyl-5,5′-dimethyl hydantoin (CEDMH) chlorination product was solved by single-crystal X-ray diffraction. The reaction was monitored, in situ and in real time, by both powder X-ray diffraction (PXRD) and Raman spectroscopy. Our kinetic data show that the reaction progress to equilibrium is similar at all milling ball sizes. The induction period is very short (between 10 and 40 s) when using 4 mm, 5 mm, 6 mm, and 8 mm balls. For the reaction performed with a 2 mm ball, a significantly longer induction period of 9 min was observed. This could indicate that an initial energy accumulation and higher mixing efficiency are necessary before the reaction starts. Using different kinetic models, we found that the amount of powder affected by critical loading conditions during individual impacts is significantly dependent on the ball size used. An almost linear correlation between the rate of the chemical transformations and the ball volume is observed.
中文翻译:

乙内酰脲的机械化学氯化反应:通过粉末 X 射线衍射和拉曼光谱进行原位实时动力学研究
机械化学已成为合成新分子的宝贵工具,尤其是在有机化学领域。在目前的工作中,我们研究了由球磨激活和驱动的N -3-乙基-5,5-二甲基乙内酰脲 (EDMH)氯化反应的动力学特征。该反应是在一个新的小型定制有机玻璃研磨罐中使用 2 毫米、4 毫米、5 毫米、6 毫米和 8 毫米球尺寸进行的。通过单晶 X 射线衍射解析了原料 EDMH 和 1-氯-3-乙基-5,5'-二甲基乙内酰脲 (CEDMH) 氯化产物的晶体结构。原位监测反应并通过粉末 X 射线衍射 (PXRD) 和拉曼光谱实时进行。我们的动力学数据表明,在所有研磨球尺寸下,平衡的反应进程是相似的。使用 4 毫米、5 毫米、6 毫米和 8 毫米球时,诱导期非常短(10 到 40 秒之间)。对于用 2 毫米球进行的反应,观察到 9 分钟的明显更长的诱导期。这可能表明在反应开始之前需要初始能量积累和更高的混合效率。使用不同的动力学模型,我们发现在单个冲击期间受临界载荷条件影响的粉末量在很大程度上取决于所使用的球尺寸。观察到化学转化率和球体积之间几乎线性相关。
更新日期:2021-09-20
中文翻译:

乙内酰脲的机械化学氯化反应:通过粉末 X 射线衍射和拉曼光谱进行原位实时动力学研究
机械化学已成为合成新分子的宝贵工具,尤其是在有机化学领域。在目前的工作中,我们研究了由球磨激活和驱动的N -3-乙基-5,5-二甲基乙内酰脲 (EDMH)氯化反应的动力学特征。该反应是在一个新的小型定制有机玻璃研磨罐中使用 2 毫米、4 毫米、5 毫米、6 毫米和 8 毫米球尺寸进行的。通过单晶 X 射线衍射解析了原料 EDMH 和 1-氯-3-乙基-5,5'-二甲基乙内酰脲 (CEDMH) 氯化产物的晶体结构。原位监测反应并通过粉末 X 射线衍射 (PXRD) 和拉曼光谱实时进行。我们的动力学数据表明,在所有研磨球尺寸下,平衡的反应进程是相似的。使用 4 毫米、5 毫米、6 毫米和 8 毫米球时,诱导期非常短(10 到 40 秒之间)。对于用 2 毫米球进行的反应,观察到 9 分钟的明显更长的诱导期。这可能表明在反应开始之前需要初始能量积累和更高的混合效率。使用不同的动力学模型,我们发现在单个冲击期间受临界载荷条件影响的粉末量在很大程度上取决于所使用的球尺寸。观察到化学转化率和球体积之间几乎线性相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号