Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood
ACS Sensors ( IF 8.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssensors.1c01183 Kaylyn K Leung 1, 2 , Alex M Downs 2, 3 , Gabriel Ortega 1, 2 , Martin Kurnik 1, 2 , Kevin W Plaxco 1, 2, 3
ACS Sensors ( IF 8.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssensors.1c01183 Kaylyn K Leung 1, 2 , Alex M Downs 2, 3 , Gabriel Ortega 1, 2 , Martin Kurnik 1, 2 , Kevin W Plaxco 1, 2, 3
Affiliation
|
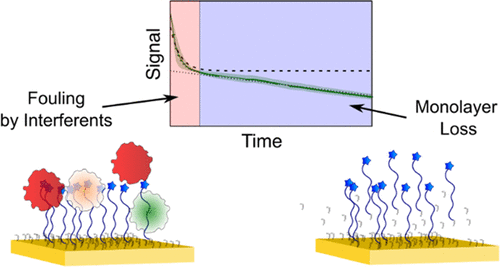
The ability to monitor drugs, metabolites, hormones, and other biomarkers in situ in the body would greatly advance both clinical practice and biomedical research. To this end, we are developing electrochemical aptamer-based (EAB) sensors, a platform technology able to perform real-time, in vivo monitoring of specific molecules irrespective of their chemical or enzymatic reactivity. An important obstacle to the deployment of EAB sensors in the challenging environments found in the living body is signal drift, whereby the sensor signal decreases over time. To date, we have demonstrated a number of approaches by which this drift can be corrected sufficiently well to achieve good measurement precision over multihour in vivo deployments. To achieve a much longer in vivo measurement duration, however, will likely require that we understand and address the sources of this effect. In response, here, we have systematically examined the mechanisms underlying the drift seen when EAB sensors and simpler, EAB-like devices are challenged in vitro at 37 °C in whole blood as a proxy for in vivo conditions. Our results demonstrate that electrochemically driven desorption of a self-assembled monolayer and fouling by blood components are the two primary sources of signal loss under these conditions, suggesting targeted approaches to remediating this degradation and thus improving the stability of EAB sensors and other, similar electrochemical biosensor technologies when deployed in the body.
中文翻译:

阐明全血中基于电化学适体的传感器信号漂移的潜在机制
在体内原位监测药物、代谢物、激素和其他生物标志物的能力将极大地促进临床实践和生物医学研究。为此,我们正在开发基于电化学适体 (EAB) 的传感器,这是一种能够对特定分子进行实时体内监测的平台技术,而不管它们的化学或酶反应性如何。在活体中发现的具有挑战性的环境中部署 EAB 传感器的一个重要障碍是信号漂移,即传感器信号随着时间的推移而降低。迄今为止,我们已经展示了多种方法,通过这些方法可以充分校正这种漂移,以在多小时的体内部署中实现良好的测量精度。然而,为了实现更长的体内测量持续时间,可能需要我们了解并解决这种影响的来源。作为回应,我们系统地研究了当 EAB 传感器和更简单的 EAB 样装置在 37°C 下在全血中作为体内条件的代表在体外受到挑战时所看到的漂移的潜在机制。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。EAB 样装置在 37 °C 的全血中进行体外挑战,作为体内条件的代表。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。EAB 样装置在 37 °C 的全血中进行体外挑战,作为体内条件的代表。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。
更新日期:2021-09-24
中文翻译:

阐明全血中基于电化学适体的传感器信号漂移的潜在机制
在体内原位监测药物、代谢物、激素和其他生物标志物的能力将极大地促进临床实践和生物医学研究。为此,我们正在开发基于电化学适体 (EAB) 的传感器,这是一种能够对特定分子进行实时体内监测的平台技术,而不管它们的化学或酶反应性如何。在活体中发现的具有挑战性的环境中部署 EAB 传感器的一个重要障碍是信号漂移,即传感器信号随着时间的推移而降低。迄今为止,我们已经展示了多种方法,通过这些方法可以充分校正这种漂移,以在多小时的体内部署中实现良好的测量精度。然而,为了实现更长的体内测量持续时间,可能需要我们了解并解决这种影响的来源。作为回应,我们系统地研究了当 EAB 传感器和更简单的 EAB 样装置在 37°C 下在全血中作为体内条件的代表在体外受到挑战时所看到的漂移的潜在机制。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。EAB 样装置在 37 °C 的全血中进行体外挑战,作为体内条件的代表。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。EAB 样装置在 37 °C 的全血中进行体外挑战,作为体内条件的代表。我们的结果表明,电化学驱动的自组装单层解吸和血液成分污染是这些条件下信号损失的两个主要来源,提出了有针对性的方法来修复这种降解,从而提高 EAB 传感器和其他类似电化学的稳定性部署在体内时的生物传感器技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号