当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Effects on Dioxygen Evolution from Ru(V)−Oxo Complexes
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-09-06 , DOI: 10.1002/ejic.202100361 Matthew T. Swann 1 , Kenneth M. Nicholas 1
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-09-06 , DOI: 10.1002/ejic.202100361 Matthew T. Swann 1 , Kenneth M. Nicholas 1
Affiliation
|
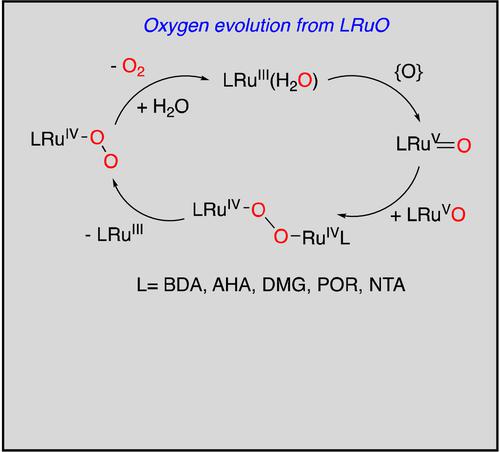
A series of ruthenium(V)−oxo compounds, LRu(V)O(n) [L=bipyridinedicarboxylate (BDA), alpha-hydroxycarboxylate (AHA), porphyrin (POR), dimethylglyoximate (DMG), and nitrilotriacetate (NTA); n=+1,0, −1] are evaluated by Density Functional Theory for their ability to produce dioxygen through coupling of Ru(V)−oxo species, bimetallic peroxides (LRu(IV)-O−O−Ru(IV)L), and dioxygen (LRu(IV)-O2) complexes. Anionic Ru−oxo complexes (AHA)2RuO− (2) and (NTA)Ru(O)Cl− (5 e) have prohibitively large free energies of coupling, while neutral and monocationic species (1 b, 3–5 a–d) show small to moderate free energies of coupling. Transition states for O−O coupling were found for (NTA)RuO (5 a), (NTA)RuO(NH3) (5 c), (NTA)RuO(Pyr) (5 d), (DMG)2ClRu(O) (8) and (POR)RuO(Cl) (9), yielding moderate activation energies in the range of 18–22 kcal/mol. The overall oxygen evolution reaction (OER) free energies decrease in favourability as the coordination number of LRuO decreases, i. e. 7>6>5. The modest activation energies and free energies along the reaction coordinate for (NTA)(L)RuO and (POR)ClRu(O) suggest that these species would undergo kinetically and thermodynamically favorable oxygen evolution.
中文翻译:

Ru(V)-Oxo 配合物对分子氧释放的结构影响
一系列钌(V)-氧代化合物,LRu(V)O (n) [L=联吡啶二羧酸盐( BDA)、α-羟基羧酸盐(AHA)、卟啉(POR)、二甲基乙醛酸盐(DMG)和次氮基三乙酸盐(NTA);n=+1,0, -1] 通过密度泛函理论评估它们通过偶联 Ru(V)-oxo 物种、双金属过氧化物 (LRu(IV)-O-O-Ru(IV)L) 产生双氧的能力) 和双氧 (LRu(IV)-O 2 ) 络合物。阴离子 Ru-oxo 配合物 (AHA) 2 RuO - ( 2 ) 和 (NTA)Ru(O)Cl - ( 5 e ) 具有非常大的耦合自由能,而中性和单阳离子物种 ( 1 b , 3 – 5 a )– d ) 显示小到中等的耦合自由能。为 (NTA)RuO ( 5 a ), (NTA)RuO(NH 3 ) ( 5 c ), (NTA)RuO(Pyr) ( 5 d ), (DMG) 2 ClRu( O) ( 8 ) 和 (POR) RuO(Cl) ( 9),产生 18-22 kcal/mol 范围内的中等活化能。随着LRuO的配位数降低,整体析氧反应(OER)自由能有利地降低,即。e. 7>6>5。(NTA)(L)RuO 和 (POR)ClRu(O) 沿着反应坐标的适度活化能和自由能表明这些物质将经历动力学和热力学有利的析氧。
更新日期:2021-09-15
中文翻译:

Ru(V)-Oxo 配合物对分子氧释放的结构影响
一系列钌(V)-氧代化合物,LRu(V)O (n) [L=联吡啶二羧酸盐( BDA)、α-羟基羧酸盐(AHA)、卟啉(POR)、二甲基乙醛酸盐(DMG)和次氮基三乙酸盐(NTA);n=+1,0, -1] 通过密度泛函理论评估它们通过偶联 Ru(V)-oxo 物种、双金属过氧化物 (LRu(IV)-O-O-Ru(IV)L) 产生双氧的能力) 和双氧 (LRu(IV)-O 2 ) 络合物。阴离子 Ru-oxo 配合物 (AHA) 2 RuO - ( 2 ) 和 (NTA)Ru(O)Cl - ( 5 e ) 具有非常大的耦合自由能,而中性和单阳离子物种 ( 1 b , 3 – 5 a )– d ) 显示小到中等的耦合自由能。为 (NTA)RuO ( 5 a ), (NTA)RuO(NH 3 ) ( 5 c ), (NTA)RuO(Pyr) ( 5 d ), (DMG) 2 ClRu( O) ( 8 ) 和 (POR) RuO(Cl) ( 9),产生 18-22 kcal/mol 范围内的中等活化能。随着LRuO的配位数降低,整体析氧反应(OER)自由能有利地降低,即。e. 7>6>5。(NTA)(L)RuO 和 (POR)ClRu(O) 沿着反应坐标的适度活化能和自由能表明这些物质将经历动力学和热力学有利的析氧。



























 京公网安备 11010802027423号
京公网安备 11010802027423号