Structure ( IF 5.7 ) Pub Date : 2021-09-06 , DOI: 10.1016/j.str.2021.08.002 Thiago V Seraphim 1 , Nardin Nano 2 , Yiu Wing Sunny Cheung 2 , Siripat Aluksanasuwan 3 , Carolina Colleti 4 , Yu-Qian Mao 2 , Vaibhav Bhandari 2 , Gavin Young 5 , Larissa Höll 6 , Sadhna Phanse 1 , Yuliya Gordiyenko 5 , Daniel R Southworth 7 , Carol V Robinson 5 , Visith Thongboonkerd 8 , Lisandra M Gava 9 , Júlio C Borges 10 , Mohan Babu 6 , Leandro R S Barbosa 11 , Carlos H I Ramos 12 , Philipp Kukura 5 , Walid A Houry 13
|
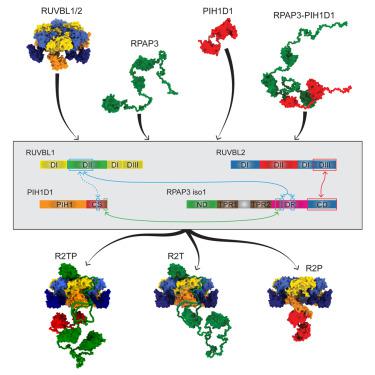
R2TP is a highly conserved chaperone complex formed by two AAA+ ATPases, RUVBL1 and RUVBL2, that associate with PIH1D1 and RPAP3 proteins. R2TP acts in promoting macromolecular complex formation. Here, we establish the principles of R2TP assembly. Three distinct RUVBL1/2-based complexes are identified: R2TP, RUVBL1/2-RPAP3 (R2T), and RUVBL1/2-PIH1D1 (R2P). Interestingly, we find that PIH1D1 does not bind to RUVBL1/RUVBL2 in R2TP and does not function as a nucleotide exchange factor; instead, RPAP3 is found to be the central subunit coordinating R2TP architecture and linking PIH1D1 and RUVBL1/2. We also report that RPAP3 contains an intrinsically disordered N-terminal domain mediating interactions with substrates whose sequences are primarily enriched for Armadillo repeat domains and other helical-type domains. Our work provides a clear and consistent model of R2TP complex structure and gives important insights into how a chaperone machine concerned with assembly of folded proteins into multisubunit complexes might work.
中文翻译:

人类 R2TP 伴侣复合物的组装原理揭示了 R2T 和 R2P 复合物的存在
R2TP 是一种高度保守的伴侣复合物,由与 PIH1D1 和 RPAP3 蛋白相关的两个 AAA+ ATP 酶 RUVBL1 和 RUVBL2 形成。R2TP 起到促进大分子复合物形成的作用。在这里,我们建立了 R2TP 组装的原则。确定了三种不同的基于 RUVBL1/2 的复合物:R2TP、RUVBL1/2-RPAP3 (R2T) 和 RUVBL1/2-PIH1D1 (R2P)。有趣的是,我们发现 PIH1D1 不与 R2TP 中的 RUVBL1/RUVBL2 结合,并且不充当核苷酸交换因子;相反,发现 RPAP3 是协调 R2TP 架构并连接 PIH1D1 和 RUVBL1/2 的中央亚基。我们还报告说,RPAP3 包含一个本质上无序的 N 端域介导与底物的相互作用,其序列主要富集犰狳重复域和其他螺旋型域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号