当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyst-free gem-chlorosulfurization of difluoromethyl-substituted diazo compounds with disulfide and PhICl2
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01422f Jiuling Li 1, 2 , Bin Li 2 , Juan Chen 2 , Xinyu Jia 2 , Min Wang 2 , Chengjun Hao 2 , Xinhua Zheng 2 , Hongmei Dai 2 , Wenhao Hu 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01422f Jiuling Li 1, 2 , Bin Li 2 , Juan Chen 2 , Xinyu Jia 2 , Min Wang 2 , Chengjun Hao 2 , Xinhua Zheng 2 , Hongmei Dai 2 , Wenhao Hu 1
Affiliation
|
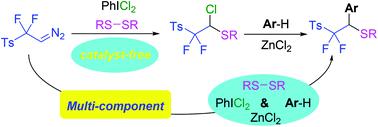
A series of gem-chlorosulfurization products bearing difluoromethyl substituents were synthesized in high to excellent yields directly from p-toluenesulfonyl difluorodiazoethane (TsCF2CHN2), disulfides and PhICl2 without any catalysts or additives. The mild reaction conditions and high functional group compatibility indicated the utility and sustainability of the method. In addition, the gem-chlorosulfurization products could be efficiently converted to sulfur-containing and aryl substituted difluoromethyl derivatives by a feasible multi-component operation.
中文翻译:

二氟甲基取代的重氮化合物与二硫化物和PhICl2的无催化剂偕氯硫化
直接由对甲苯磺酰基二氟重氮乙烷(TsCF 2 CHN 2)、二硫化物和PhICl 2合成了一系列含有二氟甲基取代基的偕氯硫化产品,收率高至极好,无需任何催化剂或添加剂。温和的反应条件和高官能团相容性表明该方法的实用性和可持续性。此外,通过可行的多组分操作,偕氯硫化产物可以有效地转化为含硫和芳基取代的二氟甲基衍生物。
更新日期:2021-09-06
中文翻译:

二氟甲基取代的重氮化合物与二硫化物和PhICl2的无催化剂偕氯硫化
直接由对甲苯磺酰基二氟重氮乙烷(TsCF 2 CHN 2)、二硫化物和PhICl 2合成了一系列含有二氟甲基取代基的偕氯硫化产品,收率高至极好,无需任何催化剂或添加剂。温和的反应条件和高官能团相容性表明该方法的实用性和可持续性。此外,通过可行的多组分操作,偕氯硫化产物可以有效地转化为含硫和芳基取代的二氟甲基衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号