当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
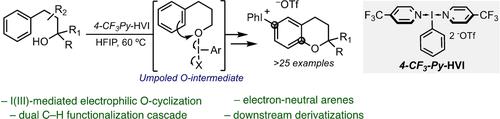
Umpolung Strategy for Arene C−H Etherification Leading to Functionalized Chromanes Enabled by I(III) N-Ligated Hypervalent Iodine Reagents
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-03 , DOI: 10.1002/adsc.202100809 Myriam Mikhael 1 , Wentao Guo 2 , Dean Tantillo 3 , Sarah Wengryniuk 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-03 , DOI: 10.1002/adsc.202100809 Myriam Mikhael 1 , Wentao Guo 2 , Dean Tantillo 3 , Sarah Wengryniuk 1
Affiliation
|
The direct formation of aryl C−O bonds via the intramolecular dehydrogenative coupling of a C−H bond and a pendant alcohol represents a powerful synthetic transformation. Herein, we report a method for intramolecular arene C−H etherification via an umpoled alcohol cyclization mediated by an I(III) N-HVI reagent. This approach provides access to functionalized chromane scaffolds from primary, secondary and tertiary alcohols via a cascade cyclization-iodonium salt formation, the latter providing a versatile functional handle for downstream derivatization. Computational studies support initial formation of an umpoled O-intermediate via I(III) ligand exchange, followed by competitive direct and spirocyclization/1,2-shift pathways.
中文翻译:

芳烃 C-H 醚化的 Umpolung 策略导致由 I(III) N-连接的高价碘试剂启用的官能化色烷
通过C-H键和侧醇的分子内脱氢偶联直接形成芳基C-O键代表了强大的合成转化。在此,我们报告了一种通过 I(III) N- HVI 试剂介导的 umpoled 醇环化进行分子内芳烃 C-H 醚化的方法。这种方法通过级联环化-碘盐形成从伯醇、仲醇和叔醇中获得官能化色满支架,后者为下游衍生化提供了通用的功能处理。计算研究支持通过 I(III) 配体交换初始形成 umpoled O-中间体,然后是竞争性直接和螺环化/1,2-转变途径。
更新日期:2021-11-09
中文翻译:

芳烃 C-H 醚化的 Umpolung 策略导致由 I(III) N-连接的高价碘试剂启用的官能化色烷
通过C-H键和侧醇的分子内脱氢偶联直接形成芳基C-O键代表了强大的合成转化。在此,我们报告了一种通过 I(III) N- HVI 试剂介导的 umpoled 醇环化进行分子内芳烃 C-H 醚化的方法。这种方法通过级联环化-碘盐形成从伯醇、仲醇和叔醇中获得官能化色满支架,后者为下游衍生化提供了通用的功能处理。计算研究支持通过 I(III) 配体交换初始形成 umpoled O-中间体,然后是竞争性直接和螺环化/1,2-转变途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号