当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidation of Dipropyl Thiosulfinate Initiated by Cl Radicals in the Gas Phase: Implications for Atmospheric Chemistry
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-09-03 , DOI: 10.1021/acsearthspacechem.1c00246 Parandaman Arathala 1 , Rabi A. Musah 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-09-03 , DOI: 10.1021/acsearthspacechem.1c00246 Parandaman Arathala 1 , Rabi A. Musah 1
Affiliation
|
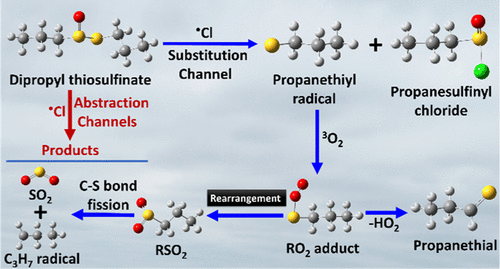
The reaction mechanism for chlorine radical (•Cl)-initiated atmospheric oxidation of dipropyl thiosulfinate (DPTS) in the gas phase was investigated using ab initio/density functional theory electronic structure calculations. The DPTS + •Cl reaction proceeds by H-abstraction and substitution pathways. The results indicate that the Cl radical adds to the S-atom of the sulfinyl [S(═O)] group of DPTS, which is followed by cleavage of the S(═O)–S single bond, leading to the formation of propanesulfinyl chloride and propanethiyl radical (PTR). The barrier height for this reaction was estimated to be −12.2 kcal mol–1 relative to the separated starting reactants. The rate coefficients were calculated for all possible H-abstraction and substitution paths using the master equation solver for the multi-energy well reactions (MESMER) kinetic code in the atmospherically relevant temperature range of 200–300 K and at 1 atm pressure. The rate coefficient data for all reaction paths indicate that the formation of propanesulfinyl chloride and PTR from the DPTS + •Cl reaction is the major path. The rate coefficient for this reaction was estimated to be ∼6.00 × 10–10 cm3 molecule–1 s–1 at 298 K and 1 atm pressure. The overall rate coefficient for the DPTS + •Cl reaction in the same temperature range was found to be ∼8.14 × 10–10 cm3 molecule–1 s–1 at 298 K. The atmospheric lifetime of DPTS was calculated to be ∼3 h in the temperatures between 200 and 300 K and at 1 atm. In addition, the branching ratio fractions for each reaction were determined and the atmospheric implications are discussed. Overall, the results reveal that while the primary products of the DPTS + Cl radical reaction are short-lived, the compounds formed from their subsequent oxidative degradation do contribute to global warming and have the potential to exhibit adverse environmental impacts.
中文翻译:

在气相中由 Cl 自由基引发的硫代亚磺酸二丙酯的氧化:对大气化学的影响
使用从头算/密度泛函理论电子结构计算研究了氯自由基 ( • Cl) 引发的硫代亚磺酸二丙酯 (DPTS) 在气相中的大气氧化反应机理。DPTS + • Cl 反应通过H-抽象和取代途径进行。结果表明,Cl 自由基与 DPTS 的亚磺酰基 [S(=O)] 基团的 S 原子相加,随后 S(=O)-S 单键断裂,导致丙亚磺酰基的形成氯和丙硫基自由基 (PTR)。该反应的势垒高度估计为 -12.2 kcal mol –1相对于分离的起始反应物。在 200-300 K 的大气相关温度范围和 1 个大气压下,使用多能井反应 (MESMER) 动力学代码的主方程求解器计算了所有可能的 H 抽象和取代路径的速率系数。所有反应路径的速率系数数据表明,DPTS + • Cl 反应生成丙亚磺酰氯和 PTR是主要路径。在 298 K 和 1 个大气压下,该反应的速率系数估计为 ~6.00 × 10 –10 cm 3分子–1 s –1。DPTS 的总速率系数 + •发现在相同温度范围内的 Cl 反应在298 K 时为 ~ 8.14 × 10 –10 cm 3分子–1 s –1。在 200 和 300 K 之间的温度下,计算出 DPTS 的大气寿命为 ~3 小时,并且在 1 个大气压。此外,确定了每个反应的支化比例,并讨论了大气影响。总体而言,结果表明,虽然 DPTS + Cl 自由基反应的主要产物寿命很短,但随后氧化降解形成的化合物确实会导致全球变暖,并有可能对环境产生不利影响。
更新日期:2021-10-22
中文翻译:

在气相中由 Cl 自由基引发的硫代亚磺酸二丙酯的氧化:对大气化学的影响
使用从头算/密度泛函理论电子结构计算研究了氯自由基 ( • Cl) 引发的硫代亚磺酸二丙酯 (DPTS) 在气相中的大气氧化反应机理。DPTS + • Cl 反应通过H-抽象和取代途径进行。结果表明,Cl 自由基与 DPTS 的亚磺酰基 [S(=O)] 基团的 S 原子相加,随后 S(=O)-S 单键断裂,导致丙亚磺酰基的形成氯和丙硫基自由基 (PTR)。该反应的势垒高度估计为 -12.2 kcal mol –1相对于分离的起始反应物。在 200-300 K 的大气相关温度范围和 1 个大气压下,使用多能井反应 (MESMER) 动力学代码的主方程求解器计算了所有可能的 H 抽象和取代路径的速率系数。所有反应路径的速率系数数据表明,DPTS + • Cl 反应生成丙亚磺酰氯和 PTR是主要路径。在 298 K 和 1 个大气压下,该反应的速率系数估计为 ~6.00 × 10 –10 cm 3分子–1 s –1。DPTS 的总速率系数 + •发现在相同温度范围内的 Cl 反应在298 K 时为 ~ 8.14 × 10 –10 cm 3分子–1 s –1。在 200 和 300 K 之间的温度下,计算出 DPTS 的大气寿命为 ~3 小时,并且在 1 个大气压。此外,确定了每个反应的支化比例,并讨论了大气影响。总体而言,结果表明,虽然 DPTS + Cl 自由基反应的主要产物寿命很短,但随后氧化降解形成的化合物确实会导致全球变暖,并有可能对环境产生不利影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号