当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
(Hetero)arylazo-1,2,3-triazoles: “Clicked” Photoswitches for Versatile Functionalization and Electronic Decoupling
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-03 , DOI: 10.1021/jacs.1c08704 Dong Fang 1 , Zhao-Yang Zhang 1 , Zhichun Shangguan 1 , Yixin He 1 , Chunyang Yu 1 , Tao Li 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-03 , DOI: 10.1021/jacs.1c08704 Dong Fang 1 , Zhao-Yang Zhang 1 , Zhichun Shangguan 1 , Yixin He 1 , Chunyang Yu 1 , Tao Li 1
Affiliation
|
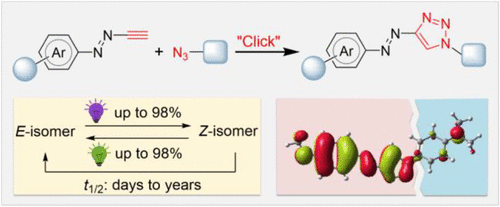
The development of light-responsive chemical systems often relies on the rational design and suitable incorporation of molecular photoswitches such as azobenzenes. Linking a photoswitch core with another π-conjugated molecular entity may give rise to intramolecular electronic coupling, which can dramatically impair the photoswitch function. Decoupling strategies have been developed based on additionally inserting a linker that can disrupt the through-bond electronic communication. Here we show that 1,2,3-triazole—a commonly used decoupling spacer—can be directly merged into the azoswitch core to construct a class of “self-decoupling” azoswitches called (hetero)arylazo-1,2,3-triazoles. Such azotriazole photoswitches are easily accessed and modularly functionalized by click chemistry. Their photoswitch property can be optimized by rational design of the substituent groups or heteroaryl rings, allowing (near-)quantitative E⇆Z photoisomerization yields and tunable Z-isomer thermal half-lives from days to years. Combined experimental and theoretical results demonstrate that the electronic structure of the photoswitch core is not substantially affected by various substituents attached to the 1,2,3-triazole unit, benefiting from its cross-conjugated nature. The combination of clickable synthesis, tunable photoswitch property, and self-decoupling ability makes (hetero)arylazo-1,2,3-triazoles intriguing molecular tools in developing photoresponsive systems with desired performance.
中文翻译:

(杂)芳基-1,2,3-三唑:用于多功能功能化和电子去耦的“点击”光电开关
光响应化学系统的发展通常依赖于分子光开关(如偶氮苯)的合理设计和适当结合。将光开关核心与另一个 π 共轭分子实体连接可能会引起分子内电子耦合,这会显着损害光开关功能。已经基于额外插入可以破坏直通电子通信的链接器开发了去耦策略。在这里,我们展示了 1,2,3-三唑——一种常用的去偶联间隔物——可以直接合并到偶氮开关核心中以构建一类称为(杂)芳基偶氮-1,2,3-三唑的“自去偶联”偶氮开关. 这种偶氮三唑光开关很容易通过点击化学访问和模块化功能化。E ⇆ Z光异构化产量和可调的Z异构体热半衰期从几天到几年不等。结合实验和理论结果表明,光开关核心的电子结构基本上不受连接到 1,2,3-三唑单元的各种取代基的影响,受益于其交叉共轭性质。可点击合成、可调光开关特性和自解耦能力的结合使(杂)芳基-1,2,3-三唑成为开发具有所需性能的光响应系统的有趣分子工具。
更新日期:2021-09-15
中文翻译:

(杂)芳基-1,2,3-三唑:用于多功能功能化和电子去耦的“点击”光电开关
光响应化学系统的发展通常依赖于分子光开关(如偶氮苯)的合理设计和适当结合。将光开关核心与另一个 π 共轭分子实体连接可能会引起分子内电子耦合,这会显着损害光开关功能。已经基于额外插入可以破坏直通电子通信的链接器开发了去耦策略。在这里,我们展示了 1,2,3-三唑——一种常用的去偶联间隔物——可以直接合并到偶氮开关核心中以构建一类称为(杂)芳基偶氮-1,2,3-三唑的“自去偶联”偶氮开关. 这种偶氮三唑光开关很容易通过点击化学访问和模块化功能化。E ⇆ Z光异构化产量和可调的Z异构体热半衰期从几天到几年不等。结合实验和理论结果表明,光开关核心的电子结构基本上不受连接到 1,2,3-三唑单元的各种取代基的影响,受益于其交叉共轭性质。可点击合成、可调光开关特性和自解耦能力的结合使(杂)芳基-1,2,3-三唑成为开发具有所需性能的光响应系统的有趣分子工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号