当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting Iminoquinones as Electrophilic at Nitrogen “N+” Synthons for C–N Bond Construction
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.orglett.1c00867 Luis M Mori-Quiroz 1 , Chelsea G Comadoll 1 , Jonathan E Super 1 , Michael D Clift 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.orglett.1c00867 Luis M Mori-Quiroz 1 , Chelsea G Comadoll 1 , Jonathan E Super 1 , Michael D Clift 1
Affiliation
|
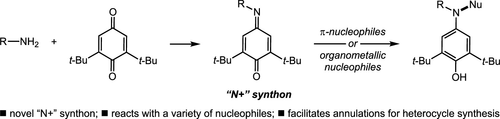
New methods for C–N bond construction exploiting the N-centered electrophilic character of iminoquinones are reported. Iminoquinones, generated in situ via the condensation of o-vinylanilines with benzoquinones, undergo acid-catalyzed cyclization to afford N-arylindoles in excellent yields. Under similar reaction conditions, homoallylic amines react analogously to afford N-arylpyrroles. Additionally, organometallic nucleophiles are shown to add to the nitrogen atom of N-alkyliminoquinones to provide amine products. Finally, iminoquinones are shown to be competent electrophiles for copper-catalyzed hydroamination.
中文翻译:

利用亚氨基醌作为氮“N+”合成子的亲电性来构建 C-N 键
报道了利用亚氨基醌的 N 中心亲电特性构建 C-N 键的新方法。通过邻乙烯基苯胺与苯醌缩合原位生成的亚氨基醌经过酸催化环化以优异的产率得到N-芳基吲哚。在类似的反应条件下,高烯丙基胺类似地反应得到N-芳基吡咯。此外,有机金属亲核试剂显示出添加到N-烷基亚氨基醌的氮原子上以提供胺产物。最后,亚氨基醌被证明是铜催化加氢胺化反应的亲电试剂。
更新日期:2021-09-17
中文翻译:

利用亚氨基醌作为氮“N+”合成子的亲电性来构建 C-N 键
报道了利用亚氨基醌的 N 中心亲电特性构建 C-N 键的新方法。通过邻乙烯基苯胺与苯醌缩合原位生成的亚氨基醌经过酸催化环化以优异的产率得到N-芳基吲哚。在类似的反应条件下,高烯丙基胺类似地反应得到N-芳基吡咯。此外,有机金属亲核试剂显示出添加到N-烷基亚氨基醌的氮原子上以提供胺产物。最后,亚氨基醌被证明是铜催化加氢胺化反应的亲电试剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号