当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed and Chiral Zinc Carboxylate-Assisted Allenylation of Benzamides via Kinetic Resolution
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.orglett.1c02398 Ruxia Mao 1 , Yanliang Zhao 2 , Xiaohan Zhu 1 , Fen Wang 1 , Wei-Qiao Deng 2 , Xingwei Li 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.orglett.1c02398 Ruxia Mao 1 , Yanliang Zhao 2 , Xiaohan Zhu 1 , Fen Wang 1 , Wei-Qiao Deng 2 , Xingwei Li 1, 2
Affiliation
|
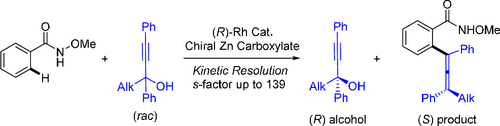
Enantioenriched allenes are important building blocks. While they have been accessed by other coupling methodologies, enantioenriched allenes have been rarely obtained via C–H activation. In this work, kinetic resolution of tertiary propargyl alcohols as an allenylating reagent has been realized via rhodium(III)-catalyzed C–H allenylation of benzamides. The reaction proceeded efficiently under mild conditions, and both the allenylated products and the propargyl alcohols were obtained in high enantioselectivities with an s-factor of up to 139. The resolution results from bias of the two propargylic substituents and is assisted by a chiral zinc carboxylate additive.
中文翻译:

铑催化和手性羧酸锌辅助苯甲酰胺的动力学拆分
对映体富集的丙二烯是重要的组成部分。虽然它们已通过其他偶联方法获得,但很少通过 C-H 活化获得对映体富集的丙二烯。在这项工作中,通过铑(III)催化的苯甲酰胺的 C-H 烯丙基化实现了叔炔丙醇作为烯丙基化试剂的动力学拆分。该反应在温和的条件下有效进行,并以高对映选择性获得了s因子高达 139的烯丙基化产物和炔丙醇。分辨率来自两个炔丙基取代基的偏向性,并由手性羧酸锌辅助添加剂。
更新日期:2021-09-17
中文翻译:

铑催化和手性羧酸锌辅助苯甲酰胺的动力学拆分
对映体富集的丙二烯是重要的组成部分。虽然它们已通过其他偶联方法获得,但很少通过 C-H 活化获得对映体富集的丙二烯。在这项工作中,通过铑(III)催化的苯甲酰胺的 C-H 烯丙基化实现了叔炔丙醇作为烯丙基化试剂的动力学拆分。该反应在温和的条件下有效进行,并以高对映选择性获得了s因子高达 139的烯丙基化产物和炔丙醇。分辨率来自两个炔丙基取代基的偏向性,并由手性羧酸锌辅助添加剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号