当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Breaking the Trend: Insight into Unforeseen Reactivity of Alkynes in Cobalt-Catalyzed Weak Chelation-Assisted Regioselective C(4)–H Functionalization of 3-Pivaloyl Indole
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c02689 Shyam Kumar Banjare 1 , Tanmayee Nanda 1 , Bedadyuti Vedvyas Pati 1 , Gopal Krushna Das Adhikari 1 , Juhi Dutta 1 , Ponneri C. Ravikumar 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c02689 Shyam Kumar Banjare 1 , Tanmayee Nanda 1 , Bedadyuti Vedvyas Pati 1 , Gopal Krushna Das Adhikari 1 , Juhi Dutta 1 , Ponneri C. Ravikumar 1
Affiliation
|
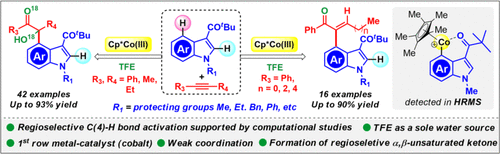
Unique reactivity of diphenylacetylene has been uncovered through weak chelation-assisted cobalt-catalyzed regioselective C(4)–H activation of 3-pivolyl indole. α-Hydroxy ketone and α,β-unsaturated ketone derivatives have been synthesized in good yields from indole and alkynes. Notably, the indole C(4)–H-functionalized α,β-unsaturated ketone product was obtained with high stereo- and regioselectivity simply by changing the coupling partner from symmetrical alkynes to unsymmetrical aromatic-aliphatic alkynes. Most importantly, trifluoroethanol acts as a sole source of water for this conversion. Quantitative detection of bis(2,2,2-trifluoroethyl) ether from dry trifluoroethanol through 19F NMR and LCMS studies indirectly confirms the in situ formation of water. A six-membered cobaltacycle intermediate was detected in HRMS, and also, this was further confirmed by the quantum mechanical calculations, which accounts for the highly regioselective C(4)–H functionalization.
中文翻译:

打破趋势:深入了解 3-新戊酰基吲哚的钴催化弱螯合辅助区域选择性 C(4)-H 官能化中炔烃的意外反应
通过弱螯合辅助钴催化的区域选择性 C(4)-H 活化 3-pivoyl indole,发现了二苯乙炔的独特反应性。α-羟基酮和α,β-不饱和酮衍生物已经以良好的收率从吲哚和炔烃中合成。值得注意的是,通过将偶联伙伴从对称炔烃变为不对称芳族-脂肪族炔烃,即可获得具有高立体选择性和区域选择性的吲哚 C(4)–H 官能化的 α,β-不饱和酮产物。最重要的是,三氟乙醇是这种转化的唯一水源。从无水三氟乙醇中通过19定量检测双(2,2,2-三氟乙基)醚F NMR 和 LCMS 研究间接证实了水的原位形成。在 HRMS 中检测到六元钴环中间体,而且量子力学计算进一步证实了这一点,这解释了高度区域选择性的 C(4)-H 功能化。
更新日期:2021-09-17
中文翻译:

打破趋势:深入了解 3-新戊酰基吲哚的钴催化弱螯合辅助区域选择性 C(4)-H 官能化中炔烃的意外反应
通过弱螯合辅助钴催化的区域选择性 C(4)-H 活化 3-pivoyl indole,发现了二苯乙炔的独特反应性。α-羟基酮和α,β-不饱和酮衍生物已经以良好的收率从吲哚和炔烃中合成。值得注意的是,通过将偶联伙伴从对称炔烃变为不对称芳族-脂肪族炔烃,即可获得具有高立体选择性和区域选择性的吲哚 C(4)–H 官能化的 α,β-不饱和酮产物。最重要的是,三氟乙醇是这种转化的唯一水源。从无水三氟乙醇中通过19定量检测双(2,2,2-三氟乙基)醚F NMR 和 LCMS 研究间接证实了水的原位形成。在 HRMS 中检测到六元钴环中间体,而且量子力学计算进一步证实了这一点,这解释了高度区域选择性的 C(4)-H 功能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号