当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight in Surface Nanotopography Driven Cellular Migration
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acsbiomaterials.1c00853 Panthihage Ruvini L Dabare 1 , Akash Bachhuka 1 , Rahul M Visalakshan 1 , Hanieh S Shirazi 1 , Kostya Ostriko 2 , Louise E Smith 3 , Krasimir Vasilev 1, 3
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acsbiomaterials.1c00853 Panthihage Ruvini L Dabare 1 , Akash Bachhuka 1 , Rahul M Visalakshan 1 , Hanieh S Shirazi 1 , Kostya Ostriko 2 , Louise E Smith 3 , Krasimir Vasilev 1, 3
Affiliation
|
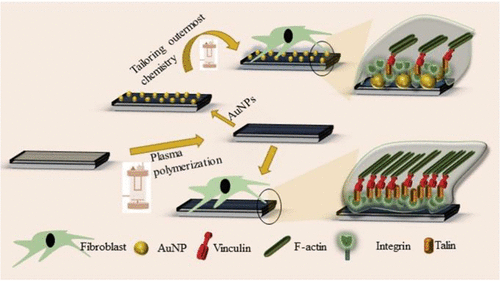
Cellular migration plays a vital role in many physiological processes. To elucidate the role of surface nanotopography on the downstream signaling pathways underlying cell migration, model surfaces having well-defined hill-like surface nanotopography and uniform surface chemistry were designed and implemented using plasma polymerization and covalent attachment of nanoparticles of predetermined size. A scratch wound assay, immunostaining, and gene expression of focal adhesion (FA) proteins were performed to determine the influence of surface nanotopography on cell migration. The results of this study demonstrate that the gap closure between cell monolayers is faster on surfaces having greater nanoscale topography. The phenomenon is predominantly driven by cell migration and was independent from cell proliferation. Qualitative and quantitative assessment of proteins involved in the signaling pathways underlying cell migration showed significant modulation by surface nanotopography. Specifically, focal adhesion sites decreased with the increase in surface nanotopography scale while the expression of FA proteins increased. This implies that nanotopography mediated modulation of cell migration is directly governed by the recruitment of receptor and adapter proteins responsible for cell-surface interaction. The results of this study indicate that biomaterial devices and constructs having rationally designed surface nanotopography and chemistry could be utilized to regulate wound healing and tissue regeneration.
中文翻译:

表面纳米拓扑驱动细胞迁移的机械洞察
细胞迁移在许多生理过程中起着至关重要的作用。为了阐明表面纳米拓扑结构在细胞迁移的下游信号通路中的作用,使用等离子体聚合和预定大小的纳米颗粒的共价连接设计并实现了具有明确定义的山丘状表面纳米拓扑结构和均匀表面化学的模型表面。进行划痕试验、免疫染色和粘着斑 (FA) 蛋白的基因表达,以确定表面纳米拓扑结构对细胞迁移的影响。这项研究的结果表明,在具有更大纳米级形貌的表面上,细胞单层之间的间隙闭合更快。这种现象主要由细胞迁移驱动,与细胞增殖无关。对参与细胞迁移信号通路的蛋白质进行的定性和定量评估表明,表面纳米形貌具有显着的调节作用。具体而言,粘着斑部位随着表面纳米形貌尺度的增加而减少,而 FA 蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。随着表面纳米形貌尺度的增加,粘着斑位点减少,而FA蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。随着表面纳米形貌尺度的增加,粘着斑位点减少,而FA蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。
更新日期:2021-10-12
中文翻译:

表面纳米拓扑驱动细胞迁移的机械洞察
细胞迁移在许多生理过程中起着至关重要的作用。为了阐明表面纳米拓扑结构在细胞迁移的下游信号通路中的作用,使用等离子体聚合和预定大小的纳米颗粒的共价连接设计并实现了具有明确定义的山丘状表面纳米拓扑结构和均匀表面化学的模型表面。进行划痕试验、免疫染色和粘着斑 (FA) 蛋白的基因表达,以确定表面纳米拓扑结构对细胞迁移的影响。这项研究的结果表明,在具有更大纳米级形貌的表面上,细胞单层之间的间隙闭合更快。这种现象主要由细胞迁移驱动,与细胞增殖无关。对参与细胞迁移信号通路的蛋白质进行的定性和定量评估表明,表面纳米形貌具有显着的调节作用。具体而言,粘着斑部位随着表面纳米形貌尺度的增加而减少,而 FA 蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。随着表面纳米形貌尺度的增加,粘着斑位点减少,而FA蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。随着表面纳米形貌尺度的增加,粘着斑位点减少,而FA蛋白的表达增加。这意味着纳米拓扑介导的细胞迁移调节直接受负责细胞表面相互作用的受体和衔接蛋白的募集控制。这项研究的结果表明,具有合理设计的表面纳米形貌和化学性质的生物材料装置和结构可用于调节伤口愈合和组织再生。


























 京公网安备 11010802027423号
京公网安备 11010802027423号