Tetrahedron ( IF 2.1 ) Pub Date : 2021-09-02 , DOI: 10.1016/j.tet.2021.132430 Lin Jiang 1 , Peiying Peng 1 , Liudong Yu 1 , Dengbang Jiang 1 , Yidan Wang 1 , Hongli Li 1 , Minglong Yuan 1 , Mingwei Yuan 1
|
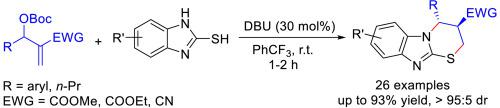
Benzimidazo [2,1-b] [1,3]thiazines as an important class of N,S-containing heterocyclic compounds are usually present in molecules with diverse biological and pharmaceutical activities. An efficient DBU-catalyzed [3 + 3] annulation reaction between Morita-Baylis-Hillman carbonates and 2-mercaptobenzimidazoles has been developed. Under mild reaction conditions, a wide range of functionalized 3,4-dihydro-2H-benzo[4,5]imidazo [2,1-b][1,3]thiazines possessing two contiguous stereogenic centers on thiazine ring were prepared in moderate to excellent yields (48–93%) and diastereoselectivities (59:41 to >95:5).
中文翻译:

通过 DBU 催化 [3+3] 环化 MBH 碳酸酯与 2 -巯基苯并咪唑
苯并咪唑 [2,1- b ] [1,3] 噻嗪类作为一类重要的含 N,S 杂环化合物,通常存在于具有多种生物和药物活性的分子中。已开发出一种有效的 DBU 催化的 [3 + 3] Morita-Baylis-Hillman 碳酸盐和 2-巯基苯并咪唑之间的环化反应。在温和的反应条件下,在噻嗪环上具有两个连续立体中心的功能化的 3,4-二氢-2 H-苯并[4,5]咪唑并 [ 2,1- b ][1,3] 噻嗪类化合物被制备成中等至优异的产率 (48–93%) 和非对映选择性 (59:41 至 >95:5)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号