当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biofilm-Mediated Immobilization of a Multienzyme Complex for Accelerating Inositol Production from Starch
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.bioconjchem.1c00338 Meixia Liu 1, 2 , Pingping Han 2 , Lingling Zhang 1, 2 , Chao Zhong 3, 4 , Chun You 1, 2, 5, 6
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.bioconjchem.1c00338 Meixia Liu 1, 2 , Pingping Han 2 , Lingling Zhang 1, 2 , Chao Zhong 3, 4 , Chun You 1, 2, 5, 6
Affiliation
|
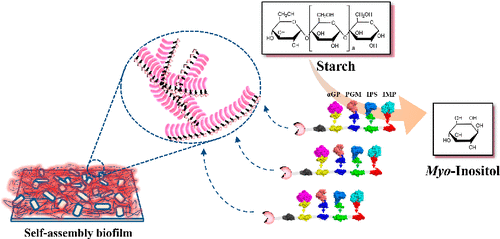
Bacterial biofilm, as a natural and renewable material, is a promising architecture for enzyme immobilization. In this study, we have demonstrated the feasibility of an Escherichia coli biofilm to immobilize a self-assembly multienzyme complex by the covalent interaction between a peptide SpyTag and its protein partner SpyCatcher. The SpyTag-labeled biofilm is displayed on the surface of E. coli by overexpressing the recombinant CsgA–SpyTag, in which CsgA is capable of forming biofilms. This SpyTag bearing biofilm is used to bind with SpyCatcher bearing synthetic mini-scaffoldin, which also contains a carbohydrate-binding module 3 (CBM3), and four different cohesins from different microorganisms. CBM3 was used to bind with cellulose, while the four different cohesins were used to recruit four dockerin-containing cascade enzymes, which were subsequently applied to convert starch to myo-inositol. Compared to the free enzyme mixture, the biofilm-immobilized enzyme complex exhibited a 4.28 times increase in initial reaction rate in producing myo-inositol from 10 g/L maltodextrin (a derivative of starch). Additionally, this biofilm-immobilized enzyme complex showed much higher recycle ability than the enzyme complex which was immobilized on a regenerated amorphous cellulose (RAC) system. In conclusion, the biofilm-mediated immobilization of the enzymatic biosystem provides a promising strategy to increase the reaction rate and enhance the stability of an in vitro enzymatic biosystem, exhibiting high potential to improve the efficiency of an in vitro biosystem.
中文翻译:

生物膜介导的多酶复合物固定化加速淀粉中的肌醇生产
细菌生物膜作为一种天然且可再生的材料,是一种很有前途的酶固定结构。在这项研究中,我们证明了大肠杆菌生物膜通过肽 SpyTag 与其蛋白质伙伴 SpyCatcher 之间的共价相互作用固定自组装多酶复合物的可行性。SpyTag 标记的生物膜显示在大肠杆菌的表面通过过表达重组 CsgA–SpyTag,其中 CsgA 能够形成生物膜。这种带有 SpyTag 的生物膜用于与带有合成微型支架的 SpyCatcher 结合,该支架还包含碳水化合物结合模块 3 (CBM3) 和来自不同微生物的四种不同的粘连蛋白。CBM3用于与纤维素结合,而四个不同的黏附被用来招4含有坞因子级联酶,其随后被施加到转化淀粉肌醇肌醇。与游离酶混合物相比,生物膜固定化酶复合物在产生肌腱时的初始反应速率提高了 4.28 倍-来自 10 g/L 麦芽糖糊精(淀粉衍生物)的肌醇。此外,与固定在再生无定形纤维素 (RAC) 系统上的酶复合物相比,这种生物膜固定化酶复合物显示出更高的回收能力。总之,生物膜介导的酶促生物系统固定化提供了一种有前景的策略,可以提高反应速率并增强体外酶促生物系统的稳定性,显示出提高体外生物系统效率的巨大潜力。
更新日期:2021-09-15
中文翻译:

生物膜介导的多酶复合物固定化加速淀粉中的肌醇生产
细菌生物膜作为一种天然且可再生的材料,是一种很有前途的酶固定结构。在这项研究中,我们证明了大肠杆菌生物膜通过肽 SpyTag 与其蛋白质伙伴 SpyCatcher 之间的共价相互作用固定自组装多酶复合物的可行性。SpyTag 标记的生物膜显示在大肠杆菌的表面通过过表达重组 CsgA–SpyTag,其中 CsgA 能够形成生物膜。这种带有 SpyTag 的生物膜用于与带有合成微型支架的 SpyCatcher 结合,该支架还包含碳水化合物结合模块 3 (CBM3) 和来自不同微生物的四种不同的粘连蛋白。CBM3用于与纤维素结合,而四个不同的黏附被用来招4含有坞因子级联酶,其随后被施加到转化淀粉肌醇肌醇。与游离酶混合物相比,生物膜固定化酶复合物在产生肌腱时的初始反应速率提高了 4.28 倍-来自 10 g/L 麦芽糖糊精(淀粉衍生物)的肌醇。此外,与固定在再生无定形纤维素 (RAC) 系统上的酶复合物相比,这种生物膜固定化酶复合物显示出更高的回收能力。总之,生物膜介导的酶促生物系统固定化提供了一种有前景的策略,可以提高反应速率并增强体外酶促生物系统的稳定性,显示出提高体外生物系统效率的巨大潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号