当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Caspase-9 Activation of Procaspase-3 but Not Procaspase-6 Is Based on the Local Context of Cleavage Site Motifs and on Sequence
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.biochem.1c00459 Ishankumar V Soni 1 , Jeanne A Hardy 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.biochem.1c00459 Ishankumar V Soni 1 , Jeanne A Hardy 1, 2
Affiliation
|
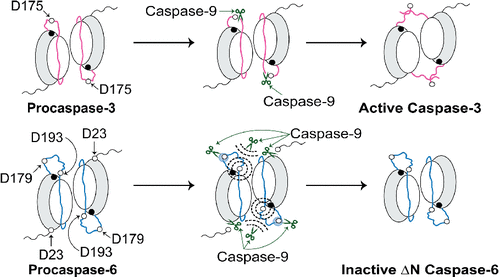
Studying the interactions between a protease and its protein substrates at a molecular level is crucial for identifying the factors facilitating selection of particular proteolytic substrates and not others. These selection criteria include both the sequence and the local context of the substrate cleavage site where the active site of the protease initially binds and then performs proteolytic cleavage. Caspase-9, an initiator of the intrinsic apoptotic pathway, mediates activation of executioner procaspase-3 by cleavage of the intersubunit linker (ISL) at site 172IETD↓S. Although procaspase-6, another executioner, possesses two ISL cleavage sites (site 1, 176DVVD↓N; site 2, 190TEVD↓A), neither is directly cut by caspase-9. Thus, caspase-9 directly activates procaspase-3 but not procaspase-6. To elucidate this selectivity of caspase-9, we engineered constructs of procaspase-3 (e.g., swapping the ISL site, 172IETD↓S, with DVVDN and TEVDA) and procaspase-6 (e.g., swapping site 1, 176DVVD↓N, and site 2, 190TEVD↓A, with IETDS). Using the substrate digestion data of these constructs, we show here that the P4–P1′ sequence of procaspase-6 ISL site 1 (DVVDN) can be accessed but not cleaved by caspase-9. We also found that caspase-9 can recognize the P4–P1′ sequence of procaspase-6 ISL site 2 (TEVDA); however, the local context of this cleavage site is the critical factor that prevents proteolytic cleavage. Overall, our data have demonstrated that both the sequence and the local context of the ISL cleavage sites play a vital role in preventing the activation of procaspase-6 directly by caspase-9.
中文翻译:

Caspase-9 激活 Procaspase-3 而不是 Procaspase-6 是基于切割位点基序的局部背景和序列
在分子水平上研究蛋白酶与其蛋白质底物之间的相互作用对于确定促进选择特定蛋白水解底物而不是其他的因素至关重要。这些选择标准包括底物切割位点的序列和局部环境,其中蛋白酶的活性位点最初结合,然后进行蛋白水解切割。Caspase-9 是内在凋亡途径的启动子,通过在位点172 IETD↓S切割亚基间接头 (ISL) 来介导执行者 procaspase-3 的激活。尽管 procaspase-6,另一个刽子手,拥有两个 ISL 切割位点(站点 1, 176 DVVD↓N;站点 2, 190TEVD↓A),两者都没有被 caspase-9 直接切割。因此,caspase-9 直接激活 procaspase-3 而不是 procaspase-6。为了阐明的caspase-9的这个选择性,我们工程改造的胱冬酶原-3的构造(例如,交换的ISL现场,172 IETD↓S,与DVVDN和TEVDA)和胱天蛋白酶原6(例如,交换位点1,176 DVVD↓N,和站点 2, 190TEVD↓A,带有 IETDS)。使用这些构建体的底物消化数据,我们在这里展示了 procaspase-6 ISL 位点 1 (DVVDN) 的 P4-P1' 序列可以访问但不能被 caspase-9 切割。我们还发现 caspase-9 可以识别 procaspase-6 ISL 位点 2 (TEVDA) 的 P4-P1' 序列;然而,该切割位点的局部环境是阻止蛋白水解切割的关键因素。总的来说,我们的数据表明,ISL 切割位点的序列和局部环境在防止 caspase-9 直接激活 procaspase-6 方面起着至关重要的作用。
更新日期:2021-09-21
中文翻译:

Caspase-9 激活 Procaspase-3 而不是 Procaspase-6 是基于切割位点基序的局部背景和序列
在分子水平上研究蛋白酶与其蛋白质底物之间的相互作用对于确定促进选择特定蛋白水解底物而不是其他的因素至关重要。这些选择标准包括底物切割位点的序列和局部环境,其中蛋白酶的活性位点最初结合,然后进行蛋白水解切割。Caspase-9 是内在凋亡途径的启动子,通过在位点172 IETD↓S切割亚基间接头 (ISL) 来介导执行者 procaspase-3 的激活。尽管 procaspase-6,另一个刽子手,拥有两个 ISL 切割位点(站点 1, 176 DVVD↓N;站点 2, 190TEVD↓A),两者都没有被 caspase-9 直接切割。因此,caspase-9 直接激活 procaspase-3 而不是 procaspase-6。为了阐明的caspase-9的这个选择性,我们工程改造的胱冬酶原-3的构造(例如,交换的ISL现场,172 IETD↓S,与DVVDN和TEVDA)和胱天蛋白酶原6(例如,交换位点1,176 DVVD↓N,和站点 2, 190TEVD↓A,带有 IETDS)。使用这些构建体的底物消化数据,我们在这里展示了 procaspase-6 ISL 位点 1 (DVVDN) 的 P4-P1' 序列可以访问但不能被 caspase-9 切割。我们还发现 caspase-9 可以识别 procaspase-6 ISL 位点 2 (TEVDA) 的 P4-P1' 序列;然而,该切割位点的局部环境是阻止蛋白水解切割的关键因素。总的来说,我们的数据表明,ISL 切割位点的序列和局部环境在防止 caspase-9 直接激活 procaspase-6 方面起着至关重要的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号