当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Luminescent Conjugated Oligothiophene h-FTAA Attenuates the Toxicity of Different Aβ Species
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.biochem.1c00265 Linnea Sandin 1 , Simon Sjödin 1 , Ann-Christin Brorsson 2 , Katarina Kågedal 1 , Livia Civitelli 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.biochem.1c00265 Linnea Sandin 1 , Simon Sjödin 1 , Ann-Christin Brorsson 2 , Katarina Kågedal 1 , Livia Civitelli 1
Affiliation
|
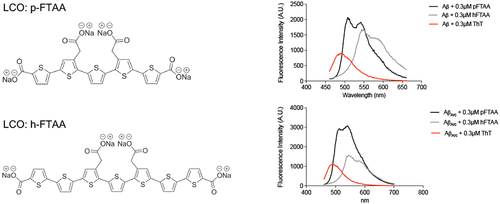
The prevailing opinion is that prefibrillar β-amyloid (Aβ) species, rather than end-stage amyloid fibrils, cause neuronal dysfunction in Alzheimer’s disease, although the mechanisms behind Aβ neurotoxicity remain to be elucidated. Luminescent conjugated oligothiophenes (LCOs) exhibit spectral properties upon binding to amyloid proteins and have previously been reported to change the toxicity of Aβ1–42 and prion protein. In a previous study, we showed that an LCO, pentamer formyl thiophene acetic acid (p-FTAA), changed the toxicity of Aβ1–42. Here we investigated whether an LCO, heptamer formyl thiophene acetic acid (h-FTAA), could change the toxicity of Aβ1–42 by comparing its behavior with that of p-FTAA. Moreover, we investigated the effects on toxicity when Aβ with the Arctic mutation (AβArc) was aggregated with both LCOs. Cell viability assays on SH-SY5Y neuroblastoma cells demonstrated that h-FTAA has a stronger impact on Aβ1–42 toxicity than does p-FTAA. Interestingly, h-FTAA, but not p-FTAA, rescued the AβArc-mediated toxicity. Aggregation kinetics and binding assay experiments with Aβ1–42 and AβArc when aggregated with both LCOs showed that h-FTAA and p-FTAA either interact with different species or affect the aggregation in different ways. In conclusion, h-FTAA protects against Aβ1–42 and AβArc toxicity, thus showing h-FTAA to be a useful tool for improving our understanding of the process of Aβ aggregation linked to cytotoxicity.
中文翻译:

发光共轭低聚噻吩 h-FTAA 减弱不同 Aβ 物种的毒性
普遍的观点是,尽管 Aβ 神经毒性背后的机制仍有待阐明,但前原纤维 β-淀粉样蛋白 (Aβ) 物种而不是终末期淀粉样蛋白原纤维会导致阿尔茨海默病的神经元功能障碍。发光的共轭低聚噻吩 (LCO) 在与淀粉样蛋白结合后表现出光谱特性,并且先前已报道可改变 Aβ 1-42和朊病毒蛋白的毒性。在之前的一项研究中,我们表明 LCO,五聚体甲酰噻吩乙酸 (p-FTAA),改变了 Aβ 1-42的毒性。在这里,我们研究了 LCO、七聚甲酰噻吩乙酸 (h-FTAA) 是否可以改变 Aβ 1-42的毒性通过将其行为与 p-FTAA 的行为进行比较。此外,我们研究了具有北极突变 (Aβ Arc ) 的Aβ与两种 LCO 聚合时对毒性的影响。SH-SY5Y 神经母细胞瘤细胞的细胞活力测定表明,h-FTAA 对 Aβ 1-42毒性的影响比 p-FTAA更强。有趣的是,h-FTAA 而不是 p-FTAA,挽救了 Aβ Arc介导的毒性。当与两种 LCO 聚合时,Aβ 1-42和 Aβ Arc 的聚合动力学和结合测定实验表明,h-FTAA 和 p-FTAA 要么与不同的物种相互作用,要么以不同的方式影响聚集。总之,h-FTAA 可防止 Aβ 1-42和 Aβ Arc 毒性,因此表明 h-FTAA 是一种有用的工具,可以提高我们对与细胞毒性相关的 Aβ 聚集过程的理解。
更新日期:2021-09-21
中文翻译:

发光共轭低聚噻吩 h-FTAA 减弱不同 Aβ 物种的毒性
普遍的观点是,尽管 Aβ 神经毒性背后的机制仍有待阐明,但前原纤维 β-淀粉样蛋白 (Aβ) 物种而不是终末期淀粉样蛋白原纤维会导致阿尔茨海默病的神经元功能障碍。发光的共轭低聚噻吩 (LCO) 在与淀粉样蛋白结合后表现出光谱特性,并且先前已报道可改变 Aβ 1-42和朊病毒蛋白的毒性。在之前的一项研究中,我们表明 LCO,五聚体甲酰噻吩乙酸 (p-FTAA),改变了 Aβ 1-42的毒性。在这里,我们研究了 LCO、七聚甲酰噻吩乙酸 (h-FTAA) 是否可以改变 Aβ 1-42的毒性通过将其行为与 p-FTAA 的行为进行比较。此外,我们研究了具有北极突变 (Aβ Arc ) 的Aβ与两种 LCO 聚合时对毒性的影响。SH-SY5Y 神经母细胞瘤细胞的细胞活力测定表明,h-FTAA 对 Aβ 1-42毒性的影响比 p-FTAA更强。有趣的是,h-FTAA 而不是 p-FTAA,挽救了 Aβ Arc介导的毒性。当与两种 LCO 聚合时,Aβ 1-42和 Aβ Arc 的聚合动力学和结合测定实验表明,h-FTAA 和 p-FTAA 要么与不同的物种相互作用,要么以不同的方式影响聚集。总之,h-FTAA 可防止 Aβ 1-42和 Aβ Arc 毒性,因此表明 h-FTAA 是一种有用的工具,可以提高我们对与细胞毒性相关的 Aβ 聚集过程的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号