当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dimeric Artesunate–Phosphatidylcholine-Based Liposomes for Irinotecan Delivery as a Combination Therapy Approach
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.molpharmaceut.1c00500 Wei He 1 , Yawei Du 1 , Tao Wang 1 , Ji Wang 1 , Lei Cheng 1 , Xinsong Li 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.molpharmaceut.1c00500 Wei He 1 , Yawei Du 1 , Tao Wang 1 , Ji Wang 1 , Lei Cheng 1 , Xinsong Li 1
Affiliation
|
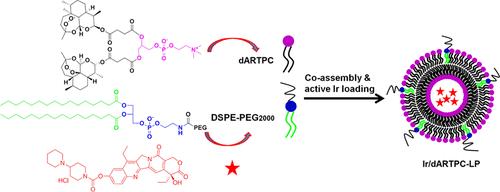
In this work, dimeric artesunate–phosphatidylcholine conjugate (dARTPC)-based liposomes encapsulated with irinotecan (Ir) were developed for anticancer combination therapy. First, dARTPC featured with unique amphipathic properties formed liposomes by classical thin-film methods. After that, Ir was encapsulated into dARTPC-based liposomes (Ir/dARTPC-LP) by the triethylammonium sucrose octasulfate gradient method. Physicochemical characterization indicated that Ir/dARTPC-LP had a mean size of around 140 nm and a negative ζ potential of approximately −30 mV. Most noticeably, liposomes displayed an encapsulation efficiency of greater than 98% with a controllable drug loading of 4–22%. The in vitro release of dihydroartemisinin (DHA) and Ir from Ir/dARTPC-LP was investigated by dialysis in different media. It was found that effective release of both DHA (65.42%) and Ir (77.28%) in a weakly acidic medium (pH 5.0) after 48 h was achieved in comparison to very slow release under a neutral environment (DHA 9.90% and Ir 8.72%), indicating the controllable release of both drugs. Confocal laser scanning microscopy confirmed the improved cellular internalization of Ir/dARTPC-LP. The cytotoxicity of Ir/dARTPC-LP was evaluated in the MCF-7, A549, and HepG2 cell lines. The results showed that Ir/dARTPC-LP had significant synergistic efficacy in the loss of cell growth. In vivo anticancer evaluation was performed using a 4T1 xenograft tumor model. Ir/dARTPC-LP had a high tumor inhibition rate of 62.7% without significant toxicity in comparison with the injection of Ir solution. Taken together, dARTPC encapsulated with Ir has great potential for anticancer combination therapy.
中文翻译:

用于伊立替康递送的二聚青蒿琥酯-磷脂酰胆碱脂质体作为一种联合治疗方法
在这项工作中,开发了用伊立替康 (Ir) 包裹的基于二聚青蒿琥酯-磷脂酰胆碱偶联物 (dARTPC) 的脂质体,用于抗癌联合治疗。首先,具有独特两亲特性的dARTPC通过经典的薄膜方法形成脂质体。之后,通过三乙基蔗糖八硫酸盐梯度法将 Ir 封装到基于 dARTPC 的脂质体 (Ir/dARTPC-LP) 中。物理化学表征表明 Ir/dARTPC-LP 的平均尺寸约为 140 nm,负 ζ 电位约为 -30 mV。最值得注意的是,脂质体的包封率大于 98%,可控载药量为 4-22%。通过在不同介质中的透析研究了 Ir/dARTPC-LP 中双氢青蒿素 (DHA) 和 Ir 的体外释放。发现在弱酸性介质 (pH 5.0) 中 48 小时后 DHA (65.42%) 和 Ir (77.28%) 的有效释放与中性环境下非常缓慢的释放 (DHA 9.90% 和 Ir 8.72) 相比%),表明两种药物的可控释放。共聚焦激光扫描显微镜证实 Ir/dARTPC-LP 的细胞内化得到改善。在 MCF-7、A549 和 HepG2 细胞系中评估了 Ir/dARTPC-LP 的细胞毒性。结果表明,Ir/dARTPC-LP 在抑制细胞生长方面具有显着的协同作用。使用 4T1 异种移植肿瘤模型进行体内抗癌评估。与注射Ir溶液相比,Ir/dARTPC-LP具有62.7%的高肿瘤抑制率且无明显毒性。综合起来,
更新日期:2021-10-04
中文翻译:

用于伊立替康递送的二聚青蒿琥酯-磷脂酰胆碱脂质体作为一种联合治疗方法
在这项工作中,开发了用伊立替康 (Ir) 包裹的基于二聚青蒿琥酯-磷脂酰胆碱偶联物 (dARTPC) 的脂质体,用于抗癌联合治疗。首先,具有独特两亲特性的dARTPC通过经典的薄膜方法形成脂质体。之后,通过三乙基蔗糖八硫酸盐梯度法将 Ir 封装到基于 dARTPC 的脂质体 (Ir/dARTPC-LP) 中。物理化学表征表明 Ir/dARTPC-LP 的平均尺寸约为 140 nm,负 ζ 电位约为 -30 mV。最值得注意的是,脂质体的包封率大于 98%,可控载药量为 4-22%。通过在不同介质中的透析研究了 Ir/dARTPC-LP 中双氢青蒿素 (DHA) 和 Ir 的体外释放。发现在弱酸性介质 (pH 5.0) 中 48 小时后 DHA (65.42%) 和 Ir (77.28%) 的有效释放与中性环境下非常缓慢的释放 (DHA 9.90% 和 Ir 8.72) 相比%),表明两种药物的可控释放。共聚焦激光扫描显微镜证实 Ir/dARTPC-LP 的细胞内化得到改善。在 MCF-7、A549 和 HepG2 细胞系中评估了 Ir/dARTPC-LP 的细胞毒性。结果表明,Ir/dARTPC-LP 在抑制细胞生长方面具有显着的协同作用。使用 4T1 异种移植肿瘤模型进行体内抗癌评估。与注射Ir溶液相比,Ir/dARTPC-LP具有62.7%的高肿瘤抑制率且无明显毒性。综合起来,



























 京公网安备 11010802027423号
京公网安备 11010802027423号