当前位置:
X-MOL 学术
›
Macromol. Theor. Simul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Initiator Structure on Thiol‐Ene Polymerization: A DFT Study
Macromolecular Theory and Simulations ( IF 1.4 ) Pub Date : 2021-09-01 , DOI: 10.1002/mats.202100040 Isa Degirmenci 1
Macromolecular Theory and Simulations ( IF 1.4 ) Pub Date : 2021-09-01 , DOI: 10.1002/mats.202100040 Isa Degirmenci 1
Affiliation
|
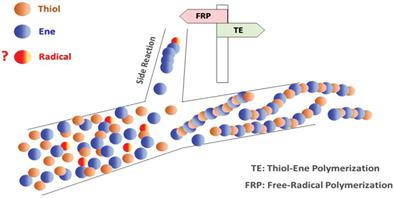
The performance of commercial initiators on thiol-ene polymerization has been investigated by utilizing the M062X/6-31++G(d,p) level of theory. The reactivity of initiators has been explained by the rate coefficients of addition (ki), which is considered side reaction and hydrogen abstraction (kHA) reactions. In this respect, radicals generated from azobisisobutyronitrile (AIBN) and 2,2-dimethoxy-2-phenylacetophenone (DMPA) have been modeled. In the case of AIBN and the propene-methyl thiol couple as reactants, it has been illustrated that side reaction is highly possible because the calculated rate coefficients ki and kHA are close to each other 1.02E+00 and 1.50E+02 l mol–1 s–1, respectively. In styrene, methyl methacrylate, and maleimide applications, the discrepancy between these reaction rates is relatively less. The high reactivity of DMPA has been explained by possessing high kHA values of formed radicals. The hydrogen abstraction reaction is significantly faster than the addition reactions for generated radicals methyl (CH3•) and benzoyl (C6H5CO•). For methyl methacrylate, side reaction is probable even in the application of DMPA since relatively fast addition reactions (the ki for CH3• and C6H5CO• radicals are 4.71E+04 and 3.42E+04 l mol–1 s–1 respectively.). Styrene and maleimide show a similar reactivity tendency with methyl methacrylate.
中文翻译:

引发剂结构对硫醇-烯聚合的影响:DFT 研究
利用 M062X/6-31++G(d,p) 理论水平研究了商业引发剂对硫醇-烯聚合的性能。引发剂的反应性可以通过加成速率系数 ( k i ) 来解释,这被认为是副反应和夺氢 ( k HA ) 反应。在这方面,已对偶氮二异丁腈 (AIBN) 和 2,2-二甲氧基-2-苯基苯乙酮 (DMPA) 产生的自由基进行了建模。在 AIBN 和丙烯-甲基硫醇对作为反应物的情况下,由于计算的速率系数k i和k HA彼此接近 1.02E+00 和 1.50E+02 l ,因此表明副反应的可能性很高摩尔–1 s –1,分别。在苯乙烯、甲基丙烯酸甲酯和马来酰亚胺应用中,这些反应速率之间的差异相对较小。DMPA 的高反应性可以通过具有高k HA值来解释形成自由基。对于生成的自由基甲基 (CH 3 •) 和苯甲酰基 (C 6 H 5 CO• ),夺氢反应明显快于加成反应。对于甲基丙烯酸甲酯,即使在 DMPA 的应用中也可能发生副反应,因为加成反应相对较快(CH 3 • 和 C 6 H 5的k iCO• 自由基分别为 4.71E+04 和 3.42E+04 l mol –1 s –1。)。苯乙烯和马来酰亚胺与甲基丙烯酸甲酯表现出相似的反应倾向。
更新日期:2021-09-01
中文翻译:

引发剂结构对硫醇-烯聚合的影响:DFT 研究
利用 M062X/6-31++G(d,p) 理论水平研究了商业引发剂对硫醇-烯聚合的性能。引发剂的反应性可以通过加成速率系数 ( k i ) 来解释,这被认为是副反应和夺氢 ( k HA ) 反应。在这方面,已对偶氮二异丁腈 (AIBN) 和 2,2-二甲氧基-2-苯基苯乙酮 (DMPA) 产生的自由基进行了建模。在 AIBN 和丙烯-甲基硫醇对作为反应物的情况下,由于计算的速率系数k i和k HA彼此接近 1.02E+00 和 1.50E+02 l ,因此表明副反应的可能性很高摩尔–1 s –1,分别。在苯乙烯、甲基丙烯酸甲酯和马来酰亚胺应用中,这些反应速率之间的差异相对较小。DMPA 的高反应性可以通过具有高k HA值来解释形成自由基。对于生成的自由基甲基 (CH 3 •) 和苯甲酰基 (C 6 H 5 CO• ),夺氢反应明显快于加成反应。对于甲基丙烯酸甲酯,即使在 DMPA 的应用中也可能发生副反应,因为加成反应相对较快(CH 3 • 和 C 6 H 5的k iCO• 自由基分别为 4.71E+04 和 3.42E+04 l mol –1 s –1。)。苯乙烯和马来酰亚胺与甲基丙烯酸甲酯表现出相似的反应倾向。


























 京公网安备 11010802027423号
京公网安备 11010802027423号