当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamic Behavior of Single-Atom Catalysts in Electrocatalysis: Identification of Cu-N3 as an Active Site for the Oxygen Reduction Reaction
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c03788 Ji Yang 1, 2, 3 , Wengang Liu 3 , Mingquan Xu 4 , Xiaoyan Liu 3 , Haifeng Qi 3 , Leilei Zhang 3 , Xiaofeng Yang 3 , Shanshan Niu 3 , Dan Zhou 3 , Yuefeng Liu 3 , Yang Su 3 , Jian-Feng Li 2 , Zhong-Qun Tian 2 , Wu Zhou 4, 5 , Aiqin Wang 1, 3 , Tao Zhang 1, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c03788 Ji Yang 1, 2, 3 , Wengang Liu 3 , Mingquan Xu 4 , Xiaoyan Liu 3 , Haifeng Qi 3 , Leilei Zhang 3 , Xiaofeng Yang 3 , Shanshan Niu 3 , Dan Zhou 3 , Yuefeng Liu 3 , Yang Su 3 , Jian-Feng Li 2 , Zhong-Qun Tian 2 , Wu Zhou 4, 5 , Aiqin Wang 1, 3 , Tao Zhang 1, 3
Affiliation
|
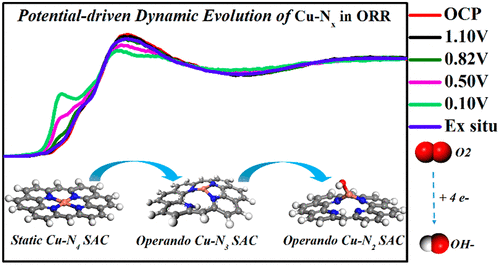
Atomically dispersed M-N-C (M refers to transition metals) materials represent the most promising catalyst alternatives to the precious metal Pt for the electrochemical reduction of oxygen (ORR), yet the genuine active sites in M-N-C remain elusive. Here, we develop a two-step approach to fabricate Cu-N-C single-atom catalysts with a uniform and well-defined Cu2+-N4 structure that exhibits comparable activity and superior durability in comparison to Pt/C. By combining operando X-ray absorption spectroscopy with theoretical calculations, we unambiguously identify the dynamic evolution of Cu-N4 to Cu-N3 and further to HO-Cu-N2 under ORR working conditions, which concurrently occurs with reduction of Cu2+ to Cu+ and is driven by the applied potential. The increase in the Cu+/Cu2+ ratio with the reduced potential indicates that the low-coordinated Cu+-N3 is the real active site, which is further supported by DFT calculations showing the lower free energy in each elemental step of the ORR on Cu+-N3 than on Cu2+-N4. These findings provide a new understanding of the dynamic electrochemistry on M-N-C catalysts and may guide the design of more efficient low-cost catalysts.
中文翻译:

单原子催化剂在电催化中的动态行为:鉴定 Cu-N3 作为氧还原反应的活性位点
原子分散的 MNC(M 指过渡金属)材料是贵金属 Pt 的最有前途的催化剂替代品,用于氧的电化学还原(ORR),但 MNC 中真正的活性位点仍然难以捉摸。在这里,我们开发了一种两步法来制造具有均匀且明确定义的 Cu 2+ -N 4结构的Cu-NC 单原子催化剂,与Pt/C 相比,该催化剂具有相当的活性和优异的耐久性。通过将操作X 射线吸收光谱与理论计算相结合,我们明确地确定了 Cu-N 4到 Cu-N 3以及进一步到 HO-Cu-N 2的动态演变ORR工作条件,其中同时进行还原的Cu发生下2+成Cu +,并通过所施加的电势来驱动。随着电位的降低,Cu + /Cu 2+比值的增加表明低配位的 Cu + -N 3是真正的活性位点,这进一步得到 DFT 计算的支持,该计算表明在每个元素步骤中较低的自由能Cu + -N 3上的ORR比Cu 2+ -N 4上的ORR 高。这些发现提供了对 MNC 催化剂动态电化学的新理解,并可能指导更有效的低成本催化剂的设计。
更新日期:2021-09-15
中文翻译:

单原子催化剂在电催化中的动态行为:鉴定 Cu-N3 作为氧还原反应的活性位点
原子分散的 MNC(M 指过渡金属)材料是贵金属 Pt 的最有前途的催化剂替代品,用于氧的电化学还原(ORR),但 MNC 中真正的活性位点仍然难以捉摸。在这里,我们开发了一种两步法来制造具有均匀且明确定义的 Cu 2+ -N 4结构的Cu-NC 单原子催化剂,与Pt/C 相比,该催化剂具有相当的活性和优异的耐久性。通过将操作X 射线吸收光谱与理论计算相结合,我们明确地确定了 Cu-N 4到 Cu-N 3以及进一步到 HO-Cu-N 2的动态演变ORR工作条件,其中同时进行还原的Cu发生下2+成Cu +,并通过所施加的电势来驱动。随着电位的降低,Cu + /Cu 2+比值的增加表明低配位的 Cu + -N 3是真正的活性位点,这进一步得到 DFT 计算的支持,该计算表明在每个元素步骤中较低的自由能Cu + -N 3上的ORR比Cu 2+ -N 4上的ORR 高。这些发现提供了对 MNC 催化剂动态电化学的新理解,并可能指导更有效的低成本催化剂的设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号