当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facilely Synthesized M-Montmorillonite (M = Cr, Fe, and Co) as Efficient Catalysts for Enhancing CO2 Desorption from Amine Solution
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.iecr.1c02487 Umair H. Bhatti 1, 2 , Wajahat W. Kazmi 1, 2 , Gwan Hong Min 1, 2 , Junaid Haider 3 , Sungchan Nam 1, 2 , Il Hyun Baek 1, 2
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.iecr.1c02487 Umair H. Bhatti 1, 2 , Wajahat W. Kazmi 1, 2 , Gwan Hong Min 1, 2 , Junaid Haider 3 , Sungchan Nam 1, 2 , Il Hyun Baek 1, 2
Affiliation
|
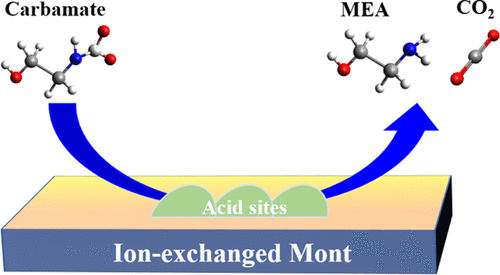
Catalytic amine regeneration has recently emerged as an effective strategy to improve CO2 desorption at low temperatures. In this work, we synthesized inexpensive M-montmorillonite (M = Cr, Fe, and Co) catalysts via a facile metal ion-exchange process and used these to optimize the CO2 desorption rate of a 30 wt % monoethanolamine (MEA) solution at a moderate temperature (∼86 °C). The metal ion-exchange process led to Si and Al leaching from the aluminosilicate layers and cation removal from the Mont interlayers, resulting in an increase in the surface acidity, mesoporosity, and total surface area of the ion-exchanged Mont catalysts. The prepared catalysts introduce acid sites to amine solution that can attach with the carbamate, carbonate, and bicarbonates, to favor the CO2 desorption at low temperatures. Overall, the CO2 desorption rate and the total amount of released CO2 were improved up to 315 and 82.5%, respectively, whereas the regeneration energy penalty was reduced by 40%, in comparison with the noncatalytic MEA solution. The impact of various physicochemical catalytic properties on the CO2 desorption performance was also evaluated. The stability of the prepared catalysts was verified in five cyclic uses and no change in the catalytic activity or structure was detected. In addition, the catalysts were readily separable by simple filtration. This work introduces an effective strategy to design abundant and cost-effective catalysts for energy-efficient CO2 capture.
中文翻译:

轻松合成 M-蒙脱石(M = Cr、Fe 和 Co)作为增强胺溶液中 CO2 解吸的有效催化剂
催化胺再生最近已成为改善低温下CO 2解吸的有效策略。在这项工作中,我们通过简便的金属离子交换工艺合成了廉价的 M-蒙脱石(M = Cr、Fe 和 Co)催化剂,并使用这些催化剂优化了 30 wt% 单乙醇胺 (MEA) 溶液的 CO 2解吸率。适中的温度(~86°C)。金属离子交换过程导致硅和铝从铝硅酸盐层中浸出,并从 Mont 中间层去除阳离子,导致离子交换 Mont 催化剂的表面酸度、介孔率和总表面积增加。制备的催化剂将酸性位点引入胺溶液中,可以与氨基甲酸酯、碳酸盐和碳酸氢盐结合,有利于 CO2低温解吸。总体而言,与非催化 MEA 溶液相比,CO 2解吸率和释放的 CO 2总量分别提高了 315% 和 82.5%,而再生能量损失降低了 40%。还评估了各种物理化学催化性能对CO 2解吸性能的影响。在五次循环使用中验证了所制备催化剂的稳定性,未检测到催化活性或结构的变化。此外,催化剂很容易通过简单的过滤分离。这项工作引入了一种有效的策略来设计丰富且具有成本效益的催化剂以实现节能的 CO 2捕获。
更新日期:2021-09-15
中文翻译:

轻松合成 M-蒙脱石(M = Cr、Fe 和 Co)作为增强胺溶液中 CO2 解吸的有效催化剂
催化胺再生最近已成为改善低温下CO 2解吸的有效策略。在这项工作中,我们通过简便的金属离子交换工艺合成了廉价的 M-蒙脱石(M = Cr、Fe 和 Co)催化剂,并使用这些催化剂优化了 30 wt% 单乙醇胺 (MEA) 溶液的 CO 2解吸率。适中的温度(~86°C)。金属离子交换过程导致硅和铝从铝硅酸盐层中浸出,并从 Mont 中间层去除阳离子,导致离子交换 Mont 催化剂的表面酸度、介孔率和总表面积增加。制备的催化剂将酸性位点引入胺溶液中,可以与氨基甲酸酯、碳酸盐和碳酸氢盐结合,有利于 CO2低温解吸。总体而言,与非催化 MEA 溶液相比,CO 2解吸率和释放的 CO 2总量分别提高了 315% 和 82.5%,而再生能量损失降低了 40%。还评估了各种物理化学催化性能对CO 2解吸性能的影响。在五次循环使用中验证了所制备催化剂的稳定性,未检测到催化活性或结构的变化。此外,催化剂很容易通过简单的过滤分离。这项工作引入了一种有效的策略来设计丰富且具有成本效益的催化剂以实现节能的 CO 2捕获。


























 京公网安备 11010802027423号
京公网安备 11010802027423号