当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The adsorption of phytate onto an Fe–Al–La trimetal composite adsorbent: kinetics, isotherms, mechanism and implication
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-08-17 , DOI: 10.1039/d1ew00318f Yuanrong Zhu 1 , Zhan Liu 2 , Kun Luo 2 , Fazhi Xie 2 , Zhongqi He 3 , Haiqing Liao 1 , John P. Giesy 1, 4, 5, 6
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-08-17 , DOI: 10.1039/d1ew00318f Yuanrong Zhu 1 , Zhan Liu 2 , Kun Luo 2 , Fazhi Xie 2 , Zhongqi He 3 , Haiqing Liao 1 , John P. Giesy 1, 4, 5, 6
Affiliation
|
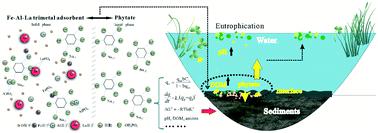
Phytate is the most abundant organic phosphorus (P) in the environment and is also an important bioavailable P source for algal blooms in some lakes. A novel Fe–Al–La (FAL) tri-metal composite adsorbent was developed by the coprecipitation method. The maximum adsorption capacity was 2.09 μmol m−2 at 298 K and an initial pH of 4.0, and it could keep high adsorption capacity when the pH varied from 4.0 to 9.0. The dominant process for the adsorption of phytate by the FAL adsorbent was surface chemical reactions mainly by monolayer coverage. The adsorption was best described by Langmuir isotherms, and its kinetics by a pseudo-second-order kinetic equation. Thermodynamic parameters indicated that adsorption of phytate by the FAL adsorbent was a spontaneous and endothermic process. The adsorption capacity decreased with pH variation from 3.2 to 11.0, especially when pH > 9.0. The sequence of strength of competition of coexisting anions with phytate was CO32− > SO42− > NO3− > Cl−. Dissolved organic matter (DOM) also competed for adsorption sites with phytate on the surface of the FAL adsorbent. Fourier transform infrared (FT-IR) spectroscopic and X-ray photoelectron spectroscopic (XPS) analyses showed that phytate had been adsorbed onto the surface of the FAL adsorbent and that Fe, Al and La all participated in adsorption. The prepared FAL adsorbent exhibited potential for removing both phytate and other phosphate species during treatment of wastewaters including those from pig and poultry manures. The FAL adsorbent could also be a potential agent for immobilization of both phytate and phosphate in overlying water and lake sediments. This study also indicated that eutrophication of lakes would increase the potential of phytate to be a bioavailable form of P in blooming of algae.
中文翻译:

植酸盐在 Fe-Al-La 三金属复合吸附剂上的吸附:动力学、等温线、机理和意义
植酸盐是环境中含量最丰富的有机磷 (P),也是一些湖泊中藻类大量繁殖的重要生物可利用磷源。通过共沉淀法开发了一种新型的 Fe-Al-La (FAL) 三金属复合吸附剂。最大吸附容量为2.09 μmol m -2在 298 K 和初始 pH 值为 4.0 时,当 pH 值从 4.0 变化到 9.0 时,它可以保持较高的吸附容量。FAL 吸附剂吸附植酸盐的主要过程是主要通过单层覆盖的表面化学反应。吸附最好用朗缪尔等温线描述,其动力学由伪二级动力学方程描述。热力学参数表明 FAL 吸附剂对植酸盐的吸附是一个自发的吸热过程。吸附容量随 pH 从 3.2 变化到 11.0 而降低,尤其是当 pH > 9.0 时。共存阴离子与植酸盐的竞争强度顺序为CO 3 2− > SO 4 2− > NO 3 − > Cl −. 溶解有机物 (DOM) 也与 FAL 吸附剂表面的植酸盐竞争吸附位点。傅里叶变换红外 (FT-IR) 光谱和 X 射线光电子光谱 (XPS) 分析表明,植酸盐已吸附在 FAL 吸附剂的表面,Fe、Al 和 La 均参与吸附。制备的 FAL 吸附剂在处理废水(包括来自猪和家禽粪便的废水)过程中表现出去除植酸盐和其他磷酸盐种类的潜力。FAL 吸附剂也可能是固定上覆水和湖泊沉积物中植酸盐和磷酸盐的潜在试剂。这项研究还表明,湖泊的富营养化会增加植酸盐成为藻类繁殖中磷的生物可利用形式的潜力。
更新日期:2021-09-01
中文翻译:

植酸盐在 Fe-Al-La 三金属复合吸附剂上的吸附:动力学、等温线、机理和意义
植酸盐是环境中含量最丰富的有机磷 (P),也是一些湖泊中藻类大量繁殖的重要生物可利用磷源。通过共沉淀法开发了一种新型的 Fe-Al-La (FAL) 三金属复合吸附剂。最大吸附容量为2.09 μmol m -2在 298 K 和初始 pH 值为 4.0 时,当 pH 值从 4.0 变化到 9.0 时,它可以保持较高的吸附容量。FAL 吸附剂吸附植酸盐的主要过程是主要通过单层覆盖的表面化学反应。吸附最好用朗缪尔等温线描述,其动力学由伪二级动力学方程描述。热力学参数表明 FAL 吸附剂对植酸盐的吸附是一个自发的吸热过程。吸附容量随 pH 从 3.2 变化到 11.0 而降低,尤其是当 pH > 9.0 时。共存阴离子与植酸盐的竞争强度顺序为CO 3 2− > SO 4 2− > NO 3 − > Cl −. 溶解有机物 (DOM) 也与 FAL 吸附剂表面的植酸盐竞争吸附位点。傅里叶变换红外 (FT-IR) 光谱和 X 射线光电子光谱 (XPS) 分析表明,植酸盐已吸附在 FAL 吸附剂的表面,Fe、Al 和 La 均参与吸附。制备的 FAL 吸附剂在处理废水(包括来自猪和家禽粪便的废水)过程中表现出去除植酸盐和其他磷酸盐种类的潜力。FAL 吸附剂也可能是固定上覆水和湖泊沉积物中植酸盐和磷酸盐的潜在试剂。这项研究还表明,湖泊的富营养化会增加植酸盐成为藻类繁殖中磷的生物可利用形式的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号