Calphad ( IF 2.4 ) Pub Date : 2021-08-31 , DOI: 10.1016/j.calphad.2021.102345 Daniel Marian Ogris 1, 2 , Ernst Gamsjäger 2
|
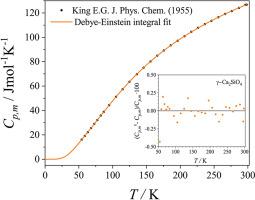
Heat capacity data for compounds located in the binary CaO–SiO2, CaO–Al2O3 and MgO–Al2O3 systems are fitted by Debye-Einstein integrals. Starting from the fitted heat capacities, the standard values of the thermodynamic functions of these compounds are calculated. In almost all cases investigated, the derived standard entropies are within the uncertainties of the values provided in literature. The Debye-Einstein coefficients obtained in this thermodynamic assessment can be used to approximate the heat capacities, enthalpies and entropies of these compounds in the temperature range from 0 to 298.15 K.
中文翻译:

由德拜-爱因斯坦积分近似的炼钢和耐火材料设计所必需的一些化合物的热容和标准熵和焓
位于二元 CaO-SiO 2、CaO-Al 2 O 3和 MgO-Al 2 O 3系统中的化合物的热容量数据由德拜-爱因斯坦积分拟合。从拟合的热容开始,计算这些化合物的热力学函数的标准值。在几乎所有调查的情况下,导出的标准熵都在文献中提供的值的不确定性范围内。在此热力学评估中获得的德拜-爱因斯坦系数可用于估算这些化合物在 0 到 298.15 K 温度范围内的热容、焓和熵。


























 京公网安备 11010802027423号
京公网安备 11010802027423号