Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.bioorg.2021.105327 Katarzyna Gałczyńska 1 , Karol Ciepluch 1 , Krystyna Kurdziel 2 , Ralf Biehl 3 , Michał Arabski 1
|
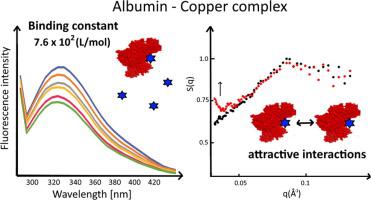
Interactions between transport proteins and compounds with therapeutic potential are pharmacologically important. In this study, using fluorescence, circular dichroism (CD), and small-angle X-ray Scattering (SAXS), we investigated the interaction between bovine serum albumin (BSA) and a copper(II)-1-allylimidazole complex with potential anti-cancer properties. The results revealed dynamic fluorescence quenching of the model carrier protein BSA by the copper(II) complex. The enthalpy change (ΔH), free energy (ΔG), and entropy change (ΔS) were calculated to be 108 kJ/mol, −16.47 kJ/mol, and 419 J/mol K, respectively, according to the Van’t Hoff equation. The reaction was an endothermic and spontaneous process, and hydrophobic interactions played a major role in binding. The results indicate a much lower affinity (Kb ∼ 102-103) for the metal complex compared with similar compounds (Kb ∼ 103-105). CD showed that the studied copper(II) complex does not change the secondary structure of the protein, while SAXS showed that the this compound may attach to the protein surface and stimulate interactions between proteins. The results suggest that the copper(II) complex with 1-allylimidazole binds weakly to BSA, leading to aggregation of albumin in solution, thereby altering its pharmacokinetic properties. The findings are pertinent to drug design.
中文翻译:

铜 (II) -1-烯丙基咪唑复合物(一种潜在的抗肿瘤剂)与牛血清白蛋白之间结合的光谱和小角 X 射线散射分析
转运蛋白和具有治疗潜力的化合物之间的相互作用在药理学上很重要。在这项研究中,我们使用荧光、圆二色性 (CD) 和小角 X 射线散射 (SAXS),研究了牛血清白蛋白 (BSA) 与具有潜在抗氧化能力的铜 (II)-1-烯丙基咪唑复合物之间的相互作用- 癌症特性。结果揭示了铜 (II) 复合物对模型载体蛋白 BSA 的动态荧光猝灭。根据 Van't Hoff 计算,焓变 (ΔH)、自由能 (ΔG) 和熵变 (ΔS) 分别为 108 kJ/mol、-16.47 kJ/mol 和 419 J/mol K方程。该反应是一个吸热和自发过程,疏水相互作用在结合中起主要作用。结果表明亲和力低得多(K b ∼ 10 2 -10 3 ) 的金属配合物与类似化合物 (K b ∼ 10 3 -10 5 ) 相比。CD 表明所研究的铜 (II) 复合物不会改变蛋白质的二级结构,而 SAXS 表明这种化合物可能附着在蛋白质表面并刺激蛋白质之间的相互作用。结果表明,铜 (II) 与 1-烯丙基咪唑的复合物与 BSA 的结合较弱,导致溶液中白蛋白的聚集,从而改变其药代动力学特性。这些发现与药物设计有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号