当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of Inflammatory and Fibrotic Encapsulation Responses of Implanted Materials with Bacterial Infection
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsbiomaterials.1c00505 Nathan A Rohner 1 , Greg D Learn 1 , Michael J Wiggins 2 , Ricky T Woofter 2 , Horst A von Recum 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsbiomaterials.1c00505 Nathan A Rohner 1 , Greg D Learn 1 , Michael J Wiggins 2 , Ricky T Woofter 2 , Horst A von Recum 1
Affiliation
|
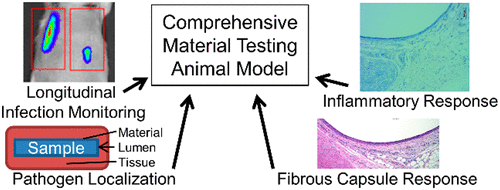
Medical device infections are costly, while preclinical assessment of antimicrobial properties for new materials is time intensive and imperfect at capturing the interrelated aspects of infection response and wound resolution. Herein, we developed an in vivo model for quantification of inflammatory and biocompatibility responses in the presence of a sustained implant-associated infection. The antimicrobial effectiveness of commercially available polymer materials was compared to that of thermoplastic polyurethane (TPU) materials modified with putative antimicrobial strategies as example test materials. Materials were incubated with bioluminescent Escherichia coli prior to implantation in a dorsal subcutaneous pocket in rats with an additional intraluminal bolus of bacteria. Infection kinetics were monitored with bioluminescence, and inflammatory infiltrate and fibrous capsule thickness were determined from stained histological sections. Our model resulted in a persistent infection, sensitive to antimicrobial effects, as the materials modified with a putative antimicrobial surface were able to significantly reduce the level of infection in animals at day 4 postimplantation with efficacy similar to that of commercially available antimicrobial drug-eluting polymers (positive controls). At day 30 postimplantation, the antimicrobial surface modified TPU tubing was found to promote complete elimination of intraluminal bacteria in the absence of antibiotics. Differences were also measurable in acute inflammation, as Wright–Giemsa staining demonstrated reduced inflammatory cell infiltration at day 4 postimplantation for antimicrobial TPU materials. Additionally, antimicrobial materials exhibited reduced fibrous capsule thickness coinciding with infection resolution, as compared to unmodified TPU controls. The developed model can be utilized for testing antimicrobial polymers in the context of a prolonged infection while also revealing concurrent differences in the infiltrating immune cell profiles and fibrous capsule thickness, thus improving the relevance of preclinical medical device material testing.
中文翻译:

细菌感染植入材料的炎症和纤维化包封反应的表征
医疗器械感染成本高昂,而新材料抗菌特性的临床前评估耗时且不完美,无法捕捉感染反应和伤口解决的相关方面。在此,我们开发了一种体内模型,用于在存在持续植入物相关感染的情况下量化炎症和生物相容性反应。将市售聚合物材料的抗菌效果与使用假定抗菌策略改性的热塑性聚氨酯 (TPU) 材料作为示例测试材料进行比较。材料与生物发光大肠杆菌一起孵育在植入大鼠的背侧皮下袋之前,具有额外的腔内细菌团块。用生物发光监测感染动力学,并从染色的组织切片确定炎症浸润和纤维囊厚度。我们的模型导致持续感染,对抗菌作用敏感,因为用假定的抗菌表面改性的材料能够在植入后第 4 天显着降低动物的感染水平,其功效与市售抗菌药物洗脱聚合物相似(阳性对照)。在植入后第 30 天,发现抗菌表面改性 TPU 管可促进在没有抗生素的情况下完全消除管腔内细菌。在急性炎症中也可以测量差异,由于 Wright-Giemsa 染色表明抗菌 TPU 材料在植入后第 4 天炎症细胞浸润减少。此外,与未改性的 TPU 对照相比,抗菌材料表现出减少的纤维胶囊厚度,这与感染消退一致。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。与未修改的 TPU 对照相比。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。与未修改的 TPU 对照相比。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。
更新日期:2021-09-13
中文翻译:

细菌感染植入材料的炎症和纤维化包封反应的表征
医疗器械感染成本高昂,而新材料抗菌特性的临床前评估耗时且不完美,无法捕捉感染反应和伤口解决的相关方面。在此,我们开发了一种体内模型,用于在存在持续植入物相关感染的情况下量化炎症和生物相容性反应。将市售聚合物材料的抗菌效果与使用假定抗菌策略改性的热塑性聚氨酯 (TPU) 材料作为示例测试材料进行比较。材料与生物发光大肠杆菌一起孵育在植入大鼠的背侧皮下袋之前,具有额外的腔内细菌团块。用生物发光监测感染动力学,并从染色的组织切片确定炎症浸润和纤维囊厚度。我们的模型导致持续感染,对抗菌作用敏感,因为用假定的抗菌表面改性的材料能够在植入后第 4 天显着降低动物的感染水平,其功效与市售抗菌药物洗脱聚合物相似(阳性对照)。在植入后第 30 天,发现抗菌表面改性 TPU 管可促进在没有抗生素的情况下完全消除管腔内细菌。在急性炎症中也可以测量差异,由于 Wright-Giemsa 染色表明抗菌 TPU 材料在植入后第 4 天炎症细胞浸润减少。此外,与未改性的 TPU 对照相比,抗菌材料表现出减少的纤维胶囊厚度,这与感染消退一致。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。与未修改的 TPU 对照相比。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。与未修改的 TPU 对照相比。开发的模型可用于在长期感染的情况下测试抗菌聚合物,同时也揭示浸润免疫细胞谱和纤维囊厚度的并发差异,从而提高临床前医疗器械材料测试的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号