当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Aminocarbonylation of Aryl Halides with N,N-Dialkylformamide Acetals
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-08-30 , DOI: 10.1002/hlca.202100162 Shuichi Hirata 1 , Takao Osako 1 , Yasuhiro Uozumi 2
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-08-30 , DOI: 10.1002/hlca.202100162 Shuichi Hirata 1 , Takao Osako 1 , Yasuhiro Uozumi 2
Affiliation
|
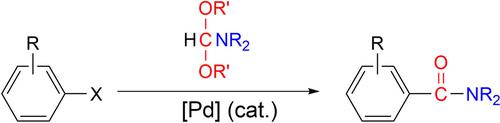
We developed a protocol for the palladium-catalyzed aminocarbonylation of aryl halides using less-toxic formamide acetals as bench-stable aminocarbonyl sources under neutral conditions. Various aryl (including heteroaryl) halides reacted with N,N-dialkylformamide acetals in the presence of a catalytic amount of tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct and xantphos to give the corresponding aromatic carboxamides at 90–140 °C without any activating agents or bases in up to quantitative chemical yield. This protocol was applied to aryl bromides, aryl iodides, and trifluoromethanesulfonic acid, as well as to relatively less-reactive aryl chlorides. A wide range of functionalities on the aromatic ring of the substrates were tolerated under the aminocarbonylation conditions. The catalytic aminocarbonylation was used to prepare the insect repellent N,N-diethyl-3-methylbenzamide as well as a synthetic intermediate of the dihydrofolate reductase inhibitor triazinate.
中文翻译:

钯催化芳基卤与 N,N-二烷基甲酰胺缩醛的氨基羰基化
我们开发了一种在中性条件下使用毒性较低的甲酰胺缩醛作为实验室稳定的氨基羰基来源的芳基卤化物的钯催化氨基羰基化的协议。在催化量的三(二亚苄基丙酮)二钯(0)-氯仿存在下,各种芳基(包括杂芳基)卤化物与N , N - 二烷基甲酰胺缩醛反应加合物和黄磷在 90–140 °C 下生成相应的芳族甲酰胺,无需任何活化剂或碱,化学产率高达定量。该方案适用于芳基溴化物、芳基碘化物和三氟甲磺酸,以及反应性相对较低的芳基氯化物。在氨基羰基化条件下,可以容忍底物芳环上的各种官能团。催化氨基羰基化反应制备了驱虫剂N , N-二乙基-3-甲基苯甲酰胺以及二氢叶酸还原酶抑制剂三嗪酸盐的合成中间体。
更新日期:2021-08-30
中文翻译:

钯催化芳基卤与 N,N-二烷基甲酰胺缩醛的氨基羰基化
我们开发了一种在中性条件下使用毒性较低的甲酰胺缩醛作为实验室稳定的氨基羰基来源的芳基卤化物的钯催化氨基羰基化的协议。在催化量的三(二亚苄基丙酮)二钯(0)-氯仿存在下,各种芳基(包括杂芳基)卤化物与N , N - 二烷基甲酰胺缩醛反应加合物和黄磷在 90–140 °C 下生成相应的芳族甲酰胺,无需任何活化剂或碱,化学产率高达定量。该方案适用于芳基溴化物、芳基碘化物和三氟甲磺酸,以及反应性相对较低的芳基氯化物。在氨基羰基化条件下,可以容忍底物芳环上的各种官能团。催化氨基羰基化反应制备了驱虫剂N , N-二乙基-3-甲基苯甲酰胺以及二氢叶酸还原酶抑制剂三嗪酸盐的合成中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号