当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Imino-1,3,4-Selenadiazoles via Tributylphosphine-Mediated Annulation of N-Aroyldiazenes with Isoselenocyanates
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-08-30 , DOI: 10.1002/adsc.202100775 Zhengyan Huang 1 , Qianqian Zhang 1 , Xiaofei Yi 1 , Zongxiang Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-08-30 , DOI: 10.1002/adsc.202100775 Zhengyan Huang 1 , Qianqian Zhang 1 , Xiaofei Yi 1 , Zongxiang Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Affiliation
|
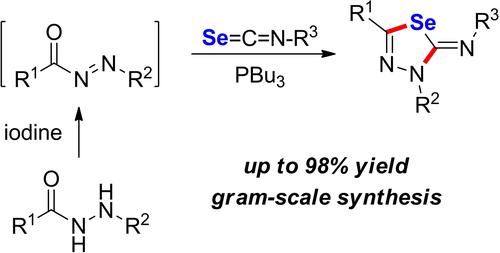
2-Imino-1,3,4-selenadiazole derivatives can be synthesized from hydrazides and isoselenocyanates by sequential oxidation and a tributylphosphine (PBu3)-promoted annulation reaction at room temperature. In this synthetic process, crude less stable N-aroyldiazene intermediates generated by I2-mediated oxidation of hydrazides were used directly in a subsequent annulation reaction to afford the selenadiazole products. The merits of the present synthetic strategy also include absence of transition metals and gram-scale synthesis.
中文翻译:

三丁基膦介导的 N-Aroyldiazenes 与异硒氰酸酯环化合成 2-Imino-1,3,4-Selenadiazoles
2-亚氨基-1,3,4-硒二唑衍生物可以在室温下通过连续氧化和三丁基膦 (PBu 3 ) 促进的环化反应从酰肼和异硒氰酸酯合成。在该合成过程中,由 I 2 -介导的酰肼氧化产生的较不稳定的粗N-芳酰基二氮中间体直接用于随后的环化反应,以提供硒二唑产物。本合成策略的优点还包括没有过渡金属和克级合成。
更新日期:2021-08-30
中文翻译:

三丁基膦介导的 N-Aroyldiazenes 与异硒氰酸酯环化合成 2-Imino-1,3,4-Selenadiazoles
2-亚氨基-1,3,4-硒二唑衍生物可以在室温下通过连续氧化和三丁基膦 (PBu 3 ) 促进的环化反应从酰肼和异硒氰酸酯合成。在该合成过程中,由 I 2 -介导的酰肼氧化产生的较不稳定的粗N-芳酰基二氮中间体直接用于随后的环化反应,以提供硒二唑产物。本合成策略的优点还包括没有过渡金属和克级合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号