当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating Twisted Amide Geometry and Reactivity Through Remote Substituent Effects
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c05854 Mizhi Xu , McKinley K. Paul , Krista K. Bullard , Christopher DuPre , Will R. Gutekunst
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c05854 Mizhi Xu , McKinley K. Paul , Krista K. Bullard , Christopher DuPre , Will R. Gutekunst
|
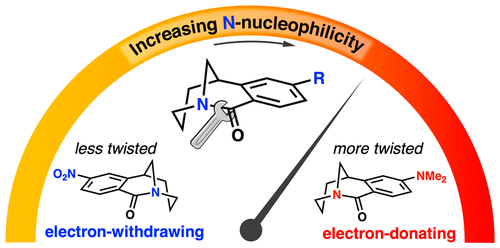
The unusual reactivity of twisted amides has long been associated with the degree of amide distortion, though classical bridged bicyclic amides offer limited methods to further modify these parameters. Here, we report that the geometry and reactivity of a single twisted amide scaffold can be significantly modulated through remote substituent effects. Guided by calculated ground state geometries, a library of twisted amide derivatives was efficiently prepared through a divergent synthetic strategy. Kinetic and mechanistic investigations of these amides in the alkylation/halide-rebound ring-opening reaction with alkyl halides show a strong positive correlation between the electron donating ability of the substituent and distortion of the amide bond, leading to rates of nucleophilic substitution spanning nearly 2 orders of magnitude. The rate limiting step of the cascade sequence is found to be dependent on the nature of the substituent, and additional studies highlight the role of solvent polarity and halide ion on reaction pathway and efficiency.
中文翻译:

通过远程取代基效应调节扭曲酰胺几何形状和反应性
长期以来,扭曲酰胺的异常反应性与酰胺变形程度有关,尽管经典的桥连双环酰胺提供了有限的方法来进一步修改这些参数。在这里,我们报告可以通过远程取代效应显着调节单个扭曲酰胺支架的几何形状和反应性。在计算的基态几何形状的指导下,通过不同的合成策略有效地制备了扭曲酰胺衍生物库。这些酰胺在烷基化/卤化物回弹开环反应中的动力学和机理研究表明,取代基的给电子能力与酰胺键的畸变之间存在很强的正相关性,导致亲核取代率跨越近 2数量级。
更新日期:2021-09-15
中文翻译:

通过远程取代基效应调节扭曲酰胺几何形状和反应性
长期以来,扭曲酰胺的异常反应性与酰胺变形程度有关,尽管经典的桥连双环酰胺提供了有限的方法来进一步修改这些参数。在这里,我们报告可以通过远程取代效应显着调节单个扭曲酰胺支架的几何形状和反应性。在计算的基态几何形状的指导下,通过不同的合成策略有效地制备了扭曲酰胺衍生物库。这些酰胺在烷基化/卤化物回弹开环反应中的动力学和机理研究表明,取代基的给电子能力与酰胺键的畸变之间存在很强的正相关性,导致亲核取代率跨越近 2数量级。


























 京公网安备 11010802027423号
京公网安备 11010802027423号