Materials Today Chemistry ( IF 7.3 ) Pub Date : 2021-08-31 , DOI: 10.1016/j.mtchem.2021.100572 Z Benková 1 , M N D S Cordeiro 2
|
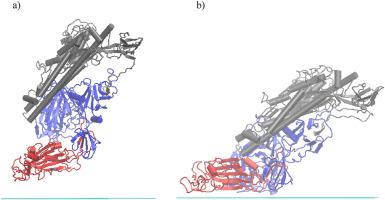
Spike glycoprotein of the SARS-CoV-2 virus and its structure play a crucial role in the infections of cells containing angiotensin-converting enzyme 2 (ACE2) as well as in the interactions of this virus with surfaces. Protection against viruses and often even their deactivation is one of the great varieties of graphene applications. The structural changes of the non-glycosylated monomer of the spike glycoprotein trimer (denoted as S-protein in this work) triggered by its adsorption onto graphene at the initial stage are investigated by means of atomistic molecular dynamics simulations. The adsorption of the S-protein happens readily during the first 10 ns. The shape of the S-protein becomes more prolate during the adsorption, but this trend, albeit less pronounced, is observed also for the freely relaxing S-protein in water. The receptor-binding domain (RBD) of the free and adsorbed S-protein manifests itself as the most rigid fragment of the whole S-protein. The adsorption even enhances the rigidity of the whole S-protein as well as its subunits. Only one residue of the RBD involved in the specific interactions with ACE2 during the cell infection is involved in the direct contact of the adsorbed S-protein with the graphene. The new intramolecular hydrogen bonds formed during the S-protein adsorption replace the S-protein-water hydrogen bonds; this trend, although less apparent, is observed also during the relaxation of the free S-protein in water. In the initial phase, the secondary structure of the RBD fragment specifically interacting with ACE2 receptor is not affected during the S-protein adsorption onto the graphene.
中文翻译:

SARS-CoV-2刺突蛋白单体在石墨烯吸附初期的结构行为
SARS-CoV-2 病毒的尖峰糖蛋白及其结构在感染含有血管紧张素转换酶 2 (ACE2) 的细胞以及该病毒与表面的相互作用中起着至关重要的作用。对病毒的保护,甚至它们的失活,是石墨烯应用的众多领域之一。通过原子分子动力学模拟研究了在初始阶段由其吸附到石墨烯上引发的刺突糖蛋白三聚体(在本工作中表示为 S 蛋白)的非糖基化单体的结构变化。S-蛋白的吸附在前 10 ns 内很容易发生。在吸附过程中,S 蛋白的形状变得更加扁平,但这种趋势虽然不那么明显,但在水中自由松弛的 S 蛋白也能观察到。游离和吸附的 S 蛋白的受体结合域 (RBD) 表现为整个 S 蛋白中最刚性的片段。吸附甚至增强了整个 S 蛋白及其亚基的刚性。在细胞感染期间,只有一个 RBD 残基参与了与 ACE2 的特定相互作用,参与了吸附的 S 蛋白与石墨烯的直接接触。S-蛋白吸附过程中形成的新分子内氢键取代了S-蛋白-水氢键;这种趋势虽然不太明显,但在水中游离 S 蛋白的松弛过程中也能观察到。在初始阶段,在 S 蛋白吸附到石墨烯上的过程中,与 ACE2 受体特异性相互作用的 RBD 片段的二级结构不受影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号