当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Construction of Single and Vicinal All-Carbon Quaternary Stereocenters through Ion-Pair-Catalyzed 1,6-Conjugate Addition
Organic Letters ( IF 5.2 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.orglett.1c02640 Yasheng Zhu 1 , Hongliang Wang 2 , Gang Wang 2 , Zehua Wang 2 , Zhaopeng Liu 1 , Lei Liu 1, 2, 3
Organic Letters ( IF 5.2 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.orglett.1c02640 Yasheng Zhu 1 , Hongliang Wang 2 , Gang Wang 2 , Zehua Wang 2 , Zhaopeng Liu 1 , Lei Liu 1, 2, 3
Affiliation
|
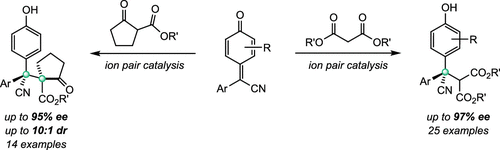
An asymmetric 1,6-conjugate addition to presynthesized δ-aryl-δ-cyano-disubstituted para-quinone methides through bifunctional phosphonium-amide-promoted ion-pair catalysis for acyclic all-carbon quaternary stereocenter construction has been described. Both acyclic and cyclic 1,3-dicarbonyls participate in the asymmetric alkylation reaction, furnishing a wide array of diarylmethanes bearing a single acyclic quaternary carbon stereocenter or vicinal cyclic and acyclic quaternary carbon stereocenters with high efficiency and excellent stereoselectivity. Computational studies elucidate the origin of the enantioselectivity.
中文翻译:

通过离子对催化的 1,6-共轭加成对单和邻位全碳四元立体中心的对映选择性构建
已经描述了通过双功能鏻-酰胺促进的离子对催化对预合成的 δ-芳基-δ-氰基-二取代的对-醌甲基化物进行不对称 1,6-共轭加成,用于非环状全碳四元立体中心结构。无环和环状 1,3-二羰基都参与不对称烷基化反应,提供了一系列具有单个无环季碳立体中心或邻环和无环季碳立体中心的二芳基甲烷,具有高效率和优异的立体选择性。计算研究阐明了对映选择性的起源。
更新日期:2021-09-17
中文翻译:

通过离子对催化的 1,6-共轭加成对单和邻位全碳四元立体中心的对映选择性构建
已经描述了通过双功能鏻-酰胺促进的离子对催化对预合成的 δ-芳基-δ-氰基-二取代的对-醌甲基化物进行不对称 1,6-共轭加成,用于非环状全碳四元立体中心结构。无环和环状 1,3-二羰基都参与不对称烷基化反应,提供了一系列具有单个无环季碳立体中心或邻环和无环季碳立体中心的二芳基甲烷,具有高效率和优异的立体选择性。计算研究阐明了对映选择性的起源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号