Biochemical and Biophysical Research Communications ( IF 3.1 ) Pub Date : 2021-08-30 , DOI: 10.1016/j.bbrc.2021.08.085 Hiroshi Sekiya 1 , Shigehiro Kamitori 2 , Hirofumi Nariya 3 , Risa Matsunami 1 , Eiji Tamai 4
|
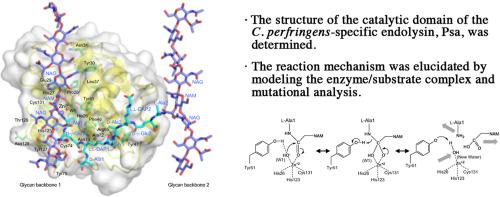
Phage-derived endolysins, enzymes that degrade peptidoglycans, have the potential to serve as alternative antimicrobial agents. Psa, which was identified as an endolysin encoded in the genome of Clostridium perfringens st13, was shown to specifically lyse C. perfringens. Psa has an N-terminal catalytic domain that is homologous to the Amidase_2 domain (PF01510), and a novel C-terminal cell wall-binding domain. Here, we determined the X-ray structure of the Psa catalytic domain (Psa-CD) at 1.65 Å resolution. Psa-CD has a typical Amidase_2 domain structure, consisting of a spherical structure with a central β-sheet surrounded by two α-helix groups. Furthermore, there is a Zn2+ at the center of Psa-CD catalytic reaction site, as well as a unique T-shaped substrate-binding groove consisting of two grooves on the molecule surface. We performed modeling study of the enzyme/substrate complex along with a mutational analysis, and demonstrated that the structure of the substrate-binding groove is closely related to the amidase activity. Furthermore, we proposed a Zn2+-mediated catalytic reaction mechanism for the Amidase_2 family, in which tyrosine constitutes part of the catalytic reaction site.
中文翻译:

产气荚膜梭菌特异性 Zn2+ 依赖性酰胺酶内溶素、Psa、催化结构域的结构和生化表征
噬菌体衍生的内溶素是降解肽聚糖的酶,有可能作为替代抗菌剂。Psa 被鉴定为在产气荚膜梭菌st13基因组中编码的细胞内溶素,显示特异性裂解产气荚膜梭菌。Psa 具有与酰胺酶_2 域 (PF01510) 同源的 N 端催化域和一个新的 C 端细胞壁结合域。在这里,我们以 1.65 Å 的分辨率确定了 Psa 催化域 (Psa-CD) 的 X 射线结构。Psa-CD 具有典型的 Amidase_2 结构域结构,由球形结构组成,中央 β-折叠被两个 α-螺旋基团包围。此外,还有一个 Zn 2+在 Psa-CD 催化反应位点的中心,还有一个独特的 T 形底物结合凹槽,由分子表面的两个凹槽组成。我们对酶/底物复合物进行了建模研究以及突变分析,并证明底物结合槽的结构与酰胺酶活性密切相关。此外,我们为 Amidase_2 家族提出了 Zn 2+介导的催化反应机制,其中酪氨酸构成催化反应位点的一部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号