当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diarylborinic Acid-Catalyzed Regioselective Ring Openings of Epoxy Alcohols with Pyrazoles, Imidazoles, Triazoles, and Other Nitrogen Heterocycles
Organic Letters ( IF 5.2 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.orglett.1c02412 Shrey P Desai 1 , Mark S Taylor 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.orglett.1c02412 Shrey P Desai 1 , Mark S Taylor 1
Affiliation
|
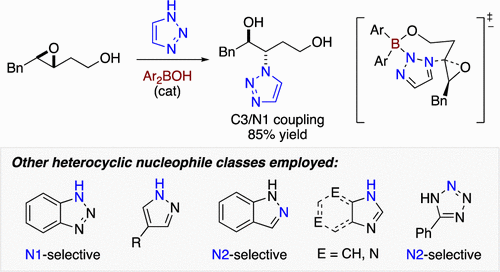
A method for regioselective ring openings of 3,4- and 2,3-epoxy alcohols with ambident nitrogen heterocycles is described. Using a diarylborinic acid catalyst, a single regioisomer is favored in couplings of nucleophile and electrophile partners that display low regioselectivity under conventional conditions. The method provides access to aromatic heterocycles bearing stereochemically defined, functionalized alkyl substituents, a product class similar in structure to medicinally relevant compounds such as the acyclic nucleoside analogues.
中文翻译:

二芳基硼酸催化环氧醇与吡唑、咪唑、三唑和其他氮杂环的区域选择性开环
描述了 3,4-和 2,3-环氧醇与双位氮杂环的区域选择性开环方法。使用二芳基硼酸催化剂,在常规条件下显示低区域选择性的亲核试剂和亲电试剂偶联物中有利于单一区域异构体。该方法提供了获得带有立体化学定义的官能化烷基取代基的芳香杂环的途径,该产品类别在结构上与医学相关化合物(如无环核苷类似物)相似。
更新日期:2021-09-17
中文翻译:

二芳基硼酸催化环氧醇与吡唑、咪唑、三唑和其他氮杂环的区域选择性开环
描述了 3,4-和 2,3-环氧醇与双位氮杂环的区域选择性开环方法。使用二芳基硼酸催化剂,在常规条件下显示低区域选择性的亲核试剂和亲电试剂偶联物中有利于单一区域异构体。该方法提供了获得带有立体化学定义的官能化烷基取代基的芳香杂环的途径,该产品类别在结构上与医学相关化合物(如无环核苷类似物)相似。


























 京公网安备 11010802027423号
京公网安备 11010802027423号