当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cerium Zirconium Solid Solution with High Faradaic Efficiency for Electrochemical Nitrogen Reduction Reaction under Ambient Condition
ChemElectroChem ( IF 4 ) Pub Date : 2021-08-29 , DOI: 10.1002/celc.202101060 Xiang Wu 1 , Xiaobo He 2 , Zhichun Li 3 , Fengxiang Yin 4
ChemElectroChem ( IF 4 ) Pub Date : 2021-08-29 , DOI: 10.1002/celc.202101060 Xiang Wu 1 , Xiaobo He 2 , Zhichun Li 3 , Fengxiang Yin 4
Affiliation
|
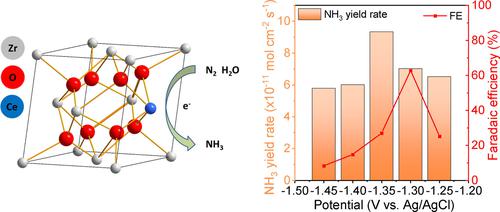
Industrial synthesis of ammonia (NH3) involves high energy consumption resulting in intense pollution. Alternatively, electrochemical nitrogen reduction reaction (NRR) to NH3 can be carried out under mild conditions, which is a new green NH3 synthesis method. Herein, cerium zirconium solid solutions (ZrxCe1-xO2) with different proportions were synthesized by NH3 precipitation method and employed as electrocatalysts for NRR. The performance of the different catalysts in 0.1 mol L−1 Na2SO4 was evaluated with H2O and N2 as reactants. The results show that Zr0.9Ce0.1O2 has the highest Faraday efficiency (FE) of 62.68 % at −1.3 V (vs. Ag/AgCl) and the highest yield rate of NH3 −9.34×10−11 mol cm−2 s−1 at −1.35 V (vs. Ag/AgCl). The impressive FE is due to more oxygen vacancies in Zr0.9Ce0.1O2 introduced by Ce3+, which is more prone to the adsorption of N2 on the catalyst surface and facilitates the electrochemical reaction.
中文翻译:

环境条件下用于电化学氮还原反应的高法拉第效率铈锆固溶体
氨(NH 3)的工业合成涉及高能耗,导致严重污染。或者,电化学氮还原反应(NRR)生成NH 3可以在温和条件下进行,这是一种新的绿色NH 3合成方法。在此,通过NH 3沉淀法合成了不同比例的铈锆固溶体(Zr x Ce 1-x O 2 ),并将其用作NRR的电催化剂。用 H 2 O 和 N 2评估了不同催化剂在 0.1 mol L -1 Na 2 SO 4中的性能作为反应物。结果表明,Zr 0.9 Ce 0.1 O 2在-1.3 V (vs. Ag/AgCl) 下具有最高的法拉第效率(FE) 62.68 % 和最高的NH 3 -9.34×10 -11 mol cm -2产率 s -1在 -1.35 V(相对于 Ag/AgCl)。令人印象深刻的FE是由于Ce 3+引入的Zr 0.9 Ce 0.1 O 2 中更多的氧空位,更容易在催化剂表面吸附N 2并促进电化学反应。
更新日期:2021-10-13
中文翻译:

环境条件下用于电化学氮还原反应的高法拉第效率铈锆固溶体
氨(NH 3)的工业合成涉及高能耗,导致严重污染。或者,电化学氮还原反应(NRR)生成NH 3可以在温和条件下进行,这是一种新的绿色NH 3合成方法。在此,通过NH 3沉淀法合成了不同比例的铈锆固溶体(Zr x Ce 1-x O 2 ),并将其用作NRR的电催化剂。用 H 2 O 和 N 2评估了不同催化剂在 0.1 mol L -1 Na 2 SO 4中的性能作为反应物。结果表明,Zr 0.9 Ce 0.1 O 2在-1.3 V (vs. Ag/AgCl) 下具有最高的法拉第效率(FE) 62.68 % 和最高的NH 3 -9.34×10 -11 mol cm -2产率 s -1在 -1.35 V(相对于 Ag/AgCl)。令人印象深刻的FE是由于Ce 3+引入的Zr 0.9 Ce 0.1 O 2 中更多的氧空位,更容易在催化剂表面吸附N 2并促进电化学反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号