Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2021-08-29 , DOI: 10.1016/j.jpha.2021.08.004 Vijay Kumar Shankar 1 , Mei Wang 2, 3 , Srinivas Ajjarapu 1 , Praveen Kolimi 1 , Bharathi Avula 3 , Reena Murthy 4 , Ikhlas Khan 3 , Sathyanarayana Narasimha Murthy 4
|
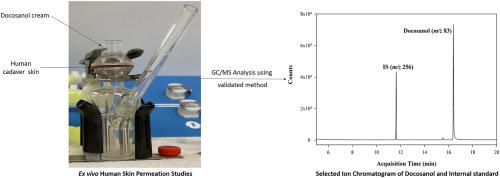
Docosanol is the only US Food and Drug Administration (FDA) approved over-the-counter topical product for treating recurrent oral-facial herpes simplex labialis. Validated analytical methods for docosanol are required to demonstrate the bioequivalence of docosanol topical products. A gas chromatography/selected ion monitoring mode mass spectrometry (GC/SIM-MS) method was developed and validated for docosanol determination in biological samples. Docosanol and isopropyl palmitate (internal standard) were separated on a high-polarity GC capillary column with (88% cyanopropy)aryl-polysiloxane employed as the stationary phase. The ions of m/z 83 and 256 were selected to monitor docosanol and isopropyl palmitate, respectively; the total run time was 20 min. The GC/SIM-MS method was validated in accordance with US FDA guidelines, and the results met the US FDA acceptance criteria. The docosanol calibration standards were linear in the 100–10000 ng/mL concentration range (R2>0.994). The recoveries for docosanol from the receptor fluid and skin homogenates were >93.2% and >95.8%, respectively. The validated method was successfully applied to analyze ex vivo human cadaver skin permeation samples. On applying Abreva® cream tube and Abreva® cream pump, the amount of docosanol that penetrated human cadaver skin at 48 h was 21.5 ± 7.01 and 24.0 ± 6.95 ng/mg, respectively. Accordingly, we concluded that the validated GC/SIM-MS was sensitive, specific, and suitable for quantifying docosanol as a quality control tool. This method can be used for routine analysis as a cost-effective alternative to other techniques.
中文翻译:

使用 GC/MS 分析二十二烷醇:方法开发、验证和在离体人体皮肤渗透研究中的应用
Docosanol 是美国食品和药物管理局 (FDA) 唯一批准用于治疗复发性口腔面部单纯性唇疱疹的非处方外用产品。需要经过验证的二十二烷醇分析方法来证明二十二烷醇外用产品的生物等效性。开发并验证了一种气相色谱/选择离子监测模式质谱 (GC/SIM-MS) 方法用于生物样品中二十二烷醇的测定。在使用(88% 氰基丙基)芳基聚硅氧烷作为固定相的高极性 GC 毛细管柱上分离二十二烷醇和棕榈酸异丙酯(内标)。m/z的离子分别选择了 83 和 256 来监测二十二烷醇和棕榈酸异丙酯;总运行时间为 20 分钟。GC/SIM-MS 方法根据美国 FDA 指南进行了验证,结果符合美国 FDA 接受标准。二十二烷醇校准标准品在 100–10000 ng/mL 浓度范围内呈线性 ( R 2>0.994)。受体液和皮肤匀浆中二十二烷醇的回收率分别为 >93.2% 和 >95.8%。经验证的方法成功应用于分析离体人体尸体皮肤渗透样品。在应用 Abreva® 奶油管和 Abreva® 奶油泵时,在 48 小时时穿透人体尸体皮肤的二十二烷醇的量分别为 21.5 ± 7.01 和 24.0 ± 6.95 ng/mg。因此,我们得出结论,经过验证的 GC/SIM-MS 灵敏、特异,适合作为质量控制工具量化二十二烷醇。该方法可用于常规分析,作为其他技术的一种经济有效的替代方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号